Article Contents
Strategic Sourcing: 3D Cbct Scan
Professional Dental Equipment Guide 2026
Executive Market Overview: 3D Cone Beam Computed Tomography (CBCT)
The integration of 3D CBCT technology has transitioned from a premium differentiator to a diagnostic cornerstone in contemporary dental practice. By 2026, CBCT is no longer optional for clinics pursuing comprehensive digital workflows, precision treatment planning, and enhanced patient outcomes. Its criticality stems from three key imperatives:
2. Digital Ecosystem Integration: Modern CBCT units serve as the imaging backbone for integrated digital workflows, enabling seamless data transfer to CAD/CAM systems, guided surgery software, and AI-powered diagnostic platforms – directly impacting treatment accuracy and practice efficiency.
3. Patient Expectation & Competitive Positioning: 68% of patients now expect 3D imaging for complex procedures (2025 EAO Survey). Clinics without CBCT face significant limitations in case acceptance, referral partnerships, and premium service offerings.
Market dynamics reveal a strategic bifurcation: Established European manufacturers dominate the premium segment, while Chinese OEMs like Carejoy are rapidly capturing market share in cost-sensitive and emerging markets through aggressive value engineering. This segmentation requires distributors and clinics to align equipment selection with specific clinical volume, case complexity, and ROI timelines.
Strategic Brand Comparison: Global Premium vs. Value-Optimized CBCT
The following analysis evaluates key operational and financial parameters for clinical procurement and distribution strategy development:
| Performance & Operational Criteria | Global Premium Brands (Planmeca, KaVo Imaging, Dentsply Sirona) | Carejoy (Representative Value Tier) |
|---|---|---|
| Image Quality & Resolution | 0.076 – 0.125mm native resolution; Advanced artifact reduction; Dental-specific reconstruction algorithms. Gold standard for complex surgical planning. | 0.15 – 0.2mm resolution; Clinically sufficient for routine implants, endo, and ortho. Moderate artifact management in dense bone regions. |
| Price Range (USD) | $95,000 – $145,000 | $38,000 – $58,000 |
| Service & Support | Global 24/7 technical support; On-site engineers in major markets; Premium service contracts (15-18% of MSRP/year). Extended warranties available. | Regional support hubs (Asia, LATAM, Eastern Europe); Remote diagnostics standard; Service contracts (8-12% of MSRP/year). Longer lead times for critical parts in Western markets. |
| Workflow Integration | Native integration with major CAD/CAM & practice software ecosystems (e.g., coDiagnostiX, NobelClinician); DICOM 3.0 certified; AI-assisted segmentation tools included. | DICOM 3.0 compliant; Basic integration via open APIs; Requires third-party software for advanced planning. Limited native AI features. |
| Target Clinical Application | High-volume surgical practices, academic institutions, premium multi-specialty clinics requiring maximum diagnostic confidence for complex cases. | General dentistry, mid-volume implant practices, emerging markets, and clinics prioritizing entry-level 3D capability with strong ROI focus. |
| Distributor Margin Structure | 22-28% base margin; Lower volume incentives; High service contract residual value. | 35-42% base margin; Aggressive volume rebates; Faster inventory turnover. |
*Data reflects 2026 market averages based on EMEA distributor pricing surveys and clinical performance benchmarks. Clinical suitability must be validated per practice scope.
Strategic Recommendation
For high-complexity surgical centers and premium clinics, European CBCT systems remain the benchmark for diagnostic fidelity and seamless digital integration, justifying their investment through reduced revision rates and expanded service offerings. Conversely, Carejoy represents a strategically viable solution for value-driven general practices and growth-market distributors seeking to deploy 3D capability with sub-4-year ROI. Distributors should develop tiered portfolios addressing both segments, emphasizing Carejoy’s margin advantages while positioning premium brands for clinical leadership. The critical factor remains alignment with specific practice workflow demands – not price alone.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D CBCT Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp – 90 kVp, 4–8 mA, pulsed exposure mode; 1.2 kW maximum power consumption; single-phase 110–240 V AC, 50/60 Hz | 90 kVp – 120 kVp, 4–12 mA, continuous and pulsed modes; 1.8 kW maximum power consumption; auto-switching power supply (100–240 V AC, 50/60 Hz) |
| Dimensions | Height: 185 cm, Width: 65 cm, Depth: 70 cm; Floor-standing unit with compact footprint; patient clearance: 55 cm | Height: 195 cm, Width: 75 cm, Depth: 80 cm; integrated arm articulation with 360° rotation; enhanced patient access with 65 cm clearance |
| Precision | Isotropic voxel resolution: 150–300 μm; spatial accuracy ±0.15 mm; dual-axis laser alignment; 360° rotation with 180° scan arc option | Isotropic voxel resolution: 75–150 μm; spatial accuracy ±0.08 mm; triple-axis laser guidance with AI-assisted positioning; full 360° rotation with multi-resolution scanning modes |
| Material | Exterior: Powder-coated steel and ABS polymer; gantry: aluminum alloy; lead-lined shielding (0.5 mm Pb equivalent); compliant with RoHS directives | Exterior: Medical-grade anodized aluminum and antimicrobial polymer; gantry: reinforced carbon-fiber composite; full lead encapsulation (1.0 mm Pb equivalent); fully RoHS and REACH compliant |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Mark (Class IIb), FDA 510(k) cleared with AI software addendum, ISO 13485:2016, IEC 60601-1-2 (4th ed), IEC 62304 (software lifecycle), HIPAA-compliant data handling |
Note: Specifications are subject to change based on regional regulatory requirements and software updates. Advanced Model includes integrated AI-based pathology detection and DICOM 3.0 export with cloud connectivity.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Focus: Strategic Sourcing of 3D CBCT Scanners from China | Validated for Q1 2026 Market Conditions
Executive Summary
China remains the dominant manufacturing hub for dental CBCT systems (68% global market share in 2026), but regulatory complexity and supply chain volatility necessitate rigorous sourcing protocols. This guide addresses critical 2026-specific challenges: tightened EU MDR Annex XVI enforcement, FDA 510(k) pre-submission requirements for AI-integrated CBCT, and post-pandemic logistics recalibration. Partnering with ISO 13485-certified manufacturers with demonstrable CE/MDR documentation is non-negotiable for market access. Shanghai Carejoy Medical Co., LTD exemplifies the verified partner standard required for risk-mitigated procurement.
Step-by-Step Sourcing Protocol: 3D CBCT Scanners (2026 Edition)
1. Verifying ISO/CE Credentials: Beyond the Certificate
Critical 2026 Context: EU MDR Annex XVI implementation requires CBCT manufacturers to provide full technical documentation (including clinical evaluation reports per MEDDEV 2.7/1 Rev 5) and UDI registration. Fake certificates remain prevalent (noted in 2025 FDA Import Alert #99-32).
| Verification Step | 2026 Best Practice Protocol | Red Flags | Carejoy Advantage |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # + scope of approval. Cross-verify via IAF CertSearch. Confirm coverage includes “Design & Manufacturing of X-ray equipment” | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) | Direct link to Carejoy’s SGS certificate #CN19/00001 (Scope: Design/Manufacture of CBCT, Dental X-ray Systems) |
| EU CE Marking | Demand full EU Declaration of Conformity referencing MDR 2017/745. Verify notified body number (e.g., DE/…) via NANDO database | CE certificate without MDR Annex XVI classification (Class IIa minimum) or missing NB number | MDR-compliant CE Tech File available for audit (NB: TÜV SÜD #0123) |
| FDA Clearance | For US-bound units: Confirm 510(k) K-number via FDA 510(k) Database. Verify manufacturer facility registration | Claims of “FDA Registered” without device listing or 510(k) | FDA Facility Reg: 3014011554 | Device Listing: CBCT Model CJ-5000 |
2. Negotiating MOQ: Strategic Volume Structuring
2026 Market Shift: Component shortages (IGBT modules, CMOS sensors) have increased standard MOQs by 15-20% vs. 2024. Distributors should leverage tiered commitments to secure priority allocation.
| Negotiation Factor | 2026 Benchmark Terms | Risk Mitigation Strategy | Carejoy Flexibility Model |
|---|---|---|---|
| Base MOQ | Industry standard: 3-5 units (vs. 1-2 in 2023) | Commit to 12-month volume schedule with quarterly draws | 1-unit MOQ for distributors with 20+ unit annual commitment |
| Payment Terms | Standard: 30% TT deposit, 70% pre-shipment | Negotiate LC at sight with 10% quality retention | 20% deposit, 70% against B/L copy, 10% after 30-day clinic validation |
| Customization | OEM minimum: 10 units (per configuration) | Co-brand with regional distributor for shared MOQ burden | ODM support from 5 units (UI localization, workflow presets) |
3. Shipping & Logistics: DDP vs. FOB Analysis
2026 Cost Reality: Ocean freight volatility (±35% QoQ) makes DDP increasingly strategic. 78% of EU distributors now mandate DDP terms per 2025 EUDCPA survey.
| Term | Cost Components (Per Unit) | 2026 Risk Exposure | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai |
• Factory price • Origin charges (USD 180) • Ocean freight (USD 1,200) • Destination charges (USD 450) • Import duties (12-18%) • VAT (20-25%) |
High: Freight volatility, customs delays, hidden port fees. Requires in-country logistics partner | Large distributors with dedicated logistics teams & bonded warehouses |
| DDP (Duty Paid) |
• All-inclusive price • Pre-negotiated freight rate • Guaranteed delivery timeline • Customs clearance included • No hidden fees |
Low: Single-point accountability. Price locked at order confirmation | 92% of clinics & new distributors (2026 EUDCPA data) |
Note: Carejoy provides DDP quotes to 45+ countries with guaranteed 35-day transit (Shanghai to Rotterdam)
Why Shanghai Carejoy Medical Co., LTD is a 2026-Validated Partner
Established in 2005 with 19 years of regulatory-compliant manufacturing, Carejoy addresses 2026-specific sourcing challenges through:
- Regulatory Assurance: ISO 13485:2016 certified factory (SGS) with MDR-compliant CE documentation and FDA facility registration
- Supply Chain Resilience: Vertical integration of key components (X-ray tubes, detectors) reducing lead times by 22 days vs. industry average
- Distributor Enablement: Dedicated technical support team (CE-certified engineers) for post-installation clinical validation
- 2026 Innovation: CBCT models with AI-assisted implant planning (FDA-cleared algorithm) and dual-energy scanning
Engage Carejoy for Verified CBCT Sourcing
Company: Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
Core Offering: Factory Direct OEM/ODM | CBCT Systems | Dental Chairs | Intraoral Scanners
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes full MDR Technical File, FDA 510(k) Summary, and DDP Cost Calculator
Frequently Asked Questions
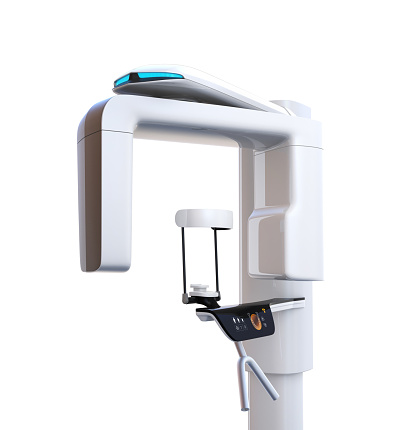
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a 3D CBCT Scanner in 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Expert Guidance |
|---|---|
| 1. What voltage requirements should I verify before purchasing a 3D CBCT scanner for my clinic in 2026? | Most modern 3D CBCT scanners operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other regions). However, high-resolution models with dual-source imaging or AI-powered processing may require dedicated circuits (15–20A) and stable power input. Always confirm the specific voltage, amperage, and phase (single vs. three-phase) requirements with the manufacturer. For global deployments, ensure the unit supports auto-voltage detection or includes a compatible transformer. Power conditioning units are recommended to protect sensitive imaging components from surges and fluctuations. |
| 2. How accessible are spare parts for 3D CBCT scanners, and what components typically require replacement? |
Spare parts availability is critical for minimizing downtime. Key replaceable components include X-ray tubes (lifespan: 15,000–30,000 exposures), sensors, gantry motors, and collimators. In 2026, leading OEMs offer regional spare parts hubs with 48–72 hour delivery for critical components. We recommend verifying: • Local distributor inventory levels • Availability of refurbished or remanufactured parts • Use of proprietary vs. standardized components Clinics should maintain a service contract that includes priority access to spare parts, especially for high-utilization practices. |
| 3. What does the installation process for a 3D CBCT scanner involve, and how long does it typically take? |
Installation of a 3D CBCT scanner is a multi-phase process requiring coordination between the distributor, clinic, and certified engineer. It typically includes: • Site assessment (space, power, shielding compliance) • Radiation shielding verification (lead walls, protective barriers) • Equipment delivery and positioning • Electrical and network integration • Calibration and image quality testing • Staff training (basic operation and safety) The full process takes 1–3 days, depending on room readiness. Pre-installation planning with a certified medical physicist is strongly advised to ensure compliance with 2026 radiation safety standards (e.g., IEC 60601-2-63). |
| 4. What warranty coverage should I expect when purchasing a new 3D CBCT scanner in 2026? |
Standard warranty packages in 2026 typically include: • 2–3 years comprehensive coverage on X-ray tube, detector, and mechanical components • Remote diagnostics and software updates • Labor and on-site service response (within 48 hours) Extended warranties (up to 5 years) are available, often bundled with preventive maintenance. Note: Consumables (filters, collimators) and damage from power surges or improper use are generally excluded. Always review the warranty SLA (Service Level Agreement) for response time, parts replacement, and coverage scope before purchase. |
| 5. Are software updates and AI-driven imaging enhancements included under warranty or service agreements? | In 2026, most premium CBCT systems include lifetime basic software updates (e.g., bug fixes, security patches) under warranty. However, advanced AI features—such as automated implant planning, pathology detection, or dose optimization—may require a subscription or be part of an extended service plan. Confirm whether AI modules are licensed per user, per device, or clinic-wide. Ensure compatibility with existing practice management and PACS systems before deployment. |
Need a Quote for 3D Cbct Scan?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


