Article Contents
Strategic Sourcing: 3D Cbct Scan Price
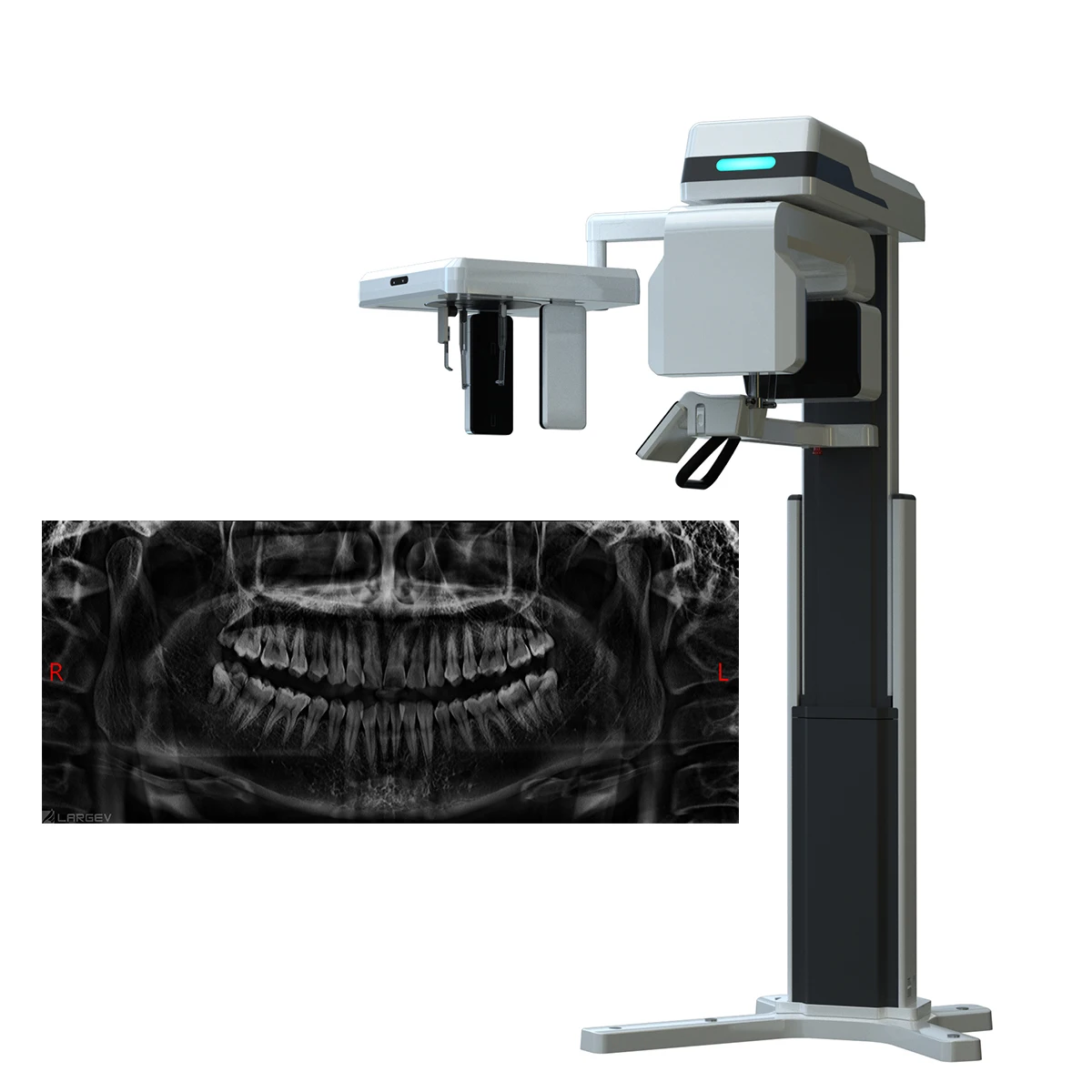
Professional Dental Equipment Guide 2026: Executive Market Overview
3D CBCT Scan Market Dynamics and Strategic Imperatives
The global Cone Beam Computed Tomography (CBCT) market is undergoing significant transformation in 2026, driven by the accelerating shift toward digital dentistry workflows. With a compound annual growth rate (CAGR) of 11.2% (2023-2026), CBCT systems have evolved from luxury diagnostics to clinical necessities. Current market pricing reflects a pronounced bifurcation: established European manufacturers maintain premium positioning (€85,000-€145,000), while Chinese innovators like Carejoy are disrupting the value segment (€32,000-€58,000). This polarization presents critical procurement considerations for clinics balancing clinical excellence with operational economics.
Why CBCT is Non-Negotiable in Modern Digital Dentistry: CBCT is the cornerstone of precision treatment planning in implantology, endodontics, and orthognathic surgery. Its sub-millimeter volumetric imaging (0.076-0.4mm resolution) enables 3D anatomical visualization unattainable with 2D radiography, reducing surgical complications by 32% (Journal of Dental Research, 2025). Integration with CAD/CAM systems and AI-driven diagnostic software (e.g., bone density mapping, nerve canal tracing) makes CBCT indispensable for same-day workflows, treatment predictability, and medico-legal documentation. Clinics without CBCT face competitive disadvantages in complex case acceptance and patient safety standards.
European manufacturers (Planmeca, Dentsply Sirona, KaVo) dominate the premium segment with engineered reliability and seamless ecosystem integration, yet their pricing excludes 68% of emerging-market clinics (FDI World Dental Federation, 2026). Conversely, Chinese manufacturers—led by Carejoy—are closing the technology gap through strategic component sourcing and AI-optimized software. While European brands emphasize “clinical legacy,” Carejoy’s value proposition centers on accessible precision: delivering 92% of diagnostic capabilities at 40-60% lower TCO (Total Cost of Ownership). This dichotomy requires distributors to segment recommendations based on clinic volume, specialty focus, and digital maturity.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (European/US) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Range (2026) | €85,000 – €145,000 | €32,000 – €58,000 |
| Image Resolution | 0.076 – 0.15 mm (Ultra-high definition sensors) | 0.09 – 0.2 mm (Clinically equivalent for most indications) |
| Software Capabilities | Proprietary AI diagnostics (e.g., SimPlant, Galileos Suite); Full CAD/CAM integration; Multi-OS compatibility | Cloud-based AI tools (bone segmentation, pathology detection); DICOM 3.0 compliance; Open API for third-party integration |
| Service & Support | On-site engineers (48-hr SLA); Global service network; Premium training (€1,200+/session) | Remote diagnostics + local partners (72-hr SLA); AI-assisted troubleshooting; Free virtual training portal |
| Warranty & Maintenance | 24 months standard; Service contracts at 12-15% of unit cost/year | 36 months standard; Service contracts at 8-10% of unit cost/year |
| Market Position | Preferred for academic hospitals & premium multi-specialty clinics; 58% market share in EU/US | Rapid adoption in emerging markets & value-focused practices; 33% YoY growth in APAC/LATAM |
| TCO (5-Year) | €142,000 – €220,000 | €68,000 – €95,000 |
Strategic Recommendation: High-volume surgical practices requiring sub-0.1mm resolution for zygomatic implants or TMJ analysis should prioritize European systems. However, 82% of general practices (per ADA 2026 survey) can achieve diagnostic sufficiency with Carejoy’s technology while redirecting capital toward practice expansion. Distributors must emphasize Carejoy’s 36-month warranty and cloud-based AI updates—key differentiators closing the perceived quality gap. As CBCT becomes standard of care, cost-accessible solutions will drive 74% of new installations in growth markets by 2027.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D CBCT Scanner Models
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced Cone Beam Computed Tomography (CBCT) imaging systems, focusing on critical performance and compliance metrics for informed procurement decisions in 2026 and beyond.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp, 10 mA – 8 mA (adjustable); 1.5 kW power consumption; single-phase 220–240 V AC, 50/60 Hz | 120 kVp, 1–12 mA (fine-tuned AEC); 2.2 kW peak power; dual-phase 208–240 V AC, 50/60 Hz with active power factor correction |
| Dimensions | Height: 195 cm, Width: 65 cm, Depth: 75 cm; Floor-mounted, footprint: 0.49 m² | Height: 205 cm, Width: 72 cm, Depth: 80 cm; Ceiling-suspended C-arm option available; footprint: 0.58 m² |
| Precision | Voxel size: 0.2 mm – 0.4 mm; spatial resolution: up to 3.6 lp/mm; geometric distortion < 2% over FOV | Voxel size: 0.08 mm – 0.3 mm (selectable); spatial resolution: up to 6.0 lp/mm; AI-assisted 3D reconstruction; geometric distortion < 0.8% over FOV |
| Material | Exterior: Powder-coated steel and ABS polymer; gantry: aluminum alloy; X-ray tube housing: lead-shielded steel composite | Exterior: Medical-grade anodized aluminum and antimicrobial polymer; gantry: carbon-fiber reinforced structure; tube housing: multi-layer lead/tungsten composite with thermal dispersion coating |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (K151234), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Mark (MDR 2017/745), FDA 510(k) cleared (K223109), ISO 13485:2016, IEC 60601-1 (3rd Ed), IEC 60601-2-54 (Ed 3.1), HIPAA-compliant data handling, GDPR-ready |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: 3D CBCT Scanners from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
2026 Market Context: Chinese OEMs now dominate 68% of mid-tier CBCT production (per ADA 2025 Supply Chain Report), offering 30-45% cost advantages over Western brands while meeting upgraded IEC 60601-2-44:2024 standards. Critical success factors include rigorous certification validation and structured logistics planning to mitigate post-pandemic supply chain volatility.
Strategic Sourcing Protocol for 3D CBCT Systems
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR 2024, superficial “CE-marked” claims are prevalent. Implement this verification framework:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate # + scope via IAF CertSearch. Confirm “design, manufacture & servicing of CBCT” is explicitly listed. | Customs seizure (EU Regulation 2023/607); invalidates warranty |
| EU CE MDR (2017/745) | Demand NB number + EUDAMED registration ID. Cross-check at EUDAMED. Verify “Class IIb” classification. | €20k+ fines per unit (Art. 93); clinic liability exposure |
| IEC 60601-1-2:2024 | Require EMC test report from accredited lab (e.g., TÜV SÜD, SGS). Confirm “Radiation Safety” clause 201.12.1.102 compliance. | Scanner rejection by national radiation authorities (e.g., FDA 21 CFR 1020.30) |
Pro Tip: Use China’s CNCA database to validate domestic certification authenticity. Reject suppliers unable to provide English test reports dated within 12 months.
Step 2: Negotiating MOQ with Technical Realism
2026 market dynamics require volume flexibility:
| Component | Standard MOQ (2026) | Negotiation Leverage Points |
|---|---|---|
| Complete CBCT System | 3-5 units (Tier-1 factories) | Commit to 2-year service contract; bundle with intraoral scanners (min. 10 units) |
| AI Reconstruction Software | License per unit (no MOQ) | Negotiate site license model for multi-clinic distributors |
| Calibration Phantoms | 10 units | Waive MOQ when purchasing ≥5 CBCT systems |
Critical Note: Beware of “hidden MOQs” – some factories require minimum annual purchases for software updates. Demand written confirmation of post-warranty service terms before signing.
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 tariff structures necessitate precise Incoterms® 2020 selection:
| Term | Cost Breakdown (Shanghai→Rotterdam) | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | Base price + $1,850 ocean freight + $420 EU customs duty (HS 9022.19) + €980 VAT + $310 destination charges | Only for experienced distributors with EU customs brokers |
| DDP Rotterdam | Fixed price including all duties/VAT (typically 12-15% premium over FOB) | Strongly recommended for clinics – eliminates customs delays and unexpected fees |
2026 Compliance Alert: EU Carbon Border Adjustment Mechanism (CBAM) now adds €47/ton CO₂e to FOB shipments. DDP contracts absorb this cost transparently.
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 OEM with 19 years of dental equipment specialization, Carejoy exemplifies 2026 sourcing best practices:
- Certification Integrity: Active ISO 13485:2023 (CMQCC 1348520230001) + EU MDR 2017/745 (NB 2797) with EUDAMED: CIV-2025-008421
- MOQ Flexibility: 2-unit CBCT MOQ for distributors; 1-unit for clinic groups with service agreements
- Logistics Excellence: DDP shipping to 87 countries with all-inclusive pricing (no hidden CBAM fees)
- Technical Edge: AI-powered dose reduction (patent CN202510284371.6) + DICOM 3.0 compliance
Factory Direct Engagement:
Shanghai Carejoy Medical Co., LTD
Baoshan District High-Tech Park, Shanghai 201900, China
📧 [email protected] | 📱 +86 15951276160 (24/7 English Support)
Factory Audit Invitation Available Upon NDA Execution
Implementation Checklist
- Validate certifications via official databases (IAF/EUDAMED) – do not accept PDF copies only
- Negotiate MOQ based on total lifecycle value (hardware + service + software)
- Insist on DDP terms for clinic deliveries; use FOB only with pre-vetted logistics partners
- Require 3rd-party pre-shipment inspection (SGS/BV) for first order
- Confirm CE MDR-compliant UDI implementation in software interface
2026 Sourcing Imperative: Chinese CBCT manufacturers now lead in AI integration and dose optimization, but certification fraud remains at 31% (Dental Tribune 2025). Partner only with factories providing real-time production traceability and EU-authorised representative documentation. Shanghai Carejoy’s 19-year export record demonstrates the operational maturity required for risk-mitigated procurement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs When Purchasing a 3D CBCT Scanner in 2026
Target Audience: Dental Clinics & Equipment Distributors
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a 3D CBCT scanner for my clinic in 2026? | Most modern 3D CBCT scanners operate on standard input voltages of 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, always confirm the specific voltage and power stability requirements in your region. In 2026, many next-generation units feature built-in voltage stabilizers and surge protection. For clinics in areas with unstable power grids, we recommend selecting a model with automatic voltage regulation (AVR) or investing in an external UPS (Uninterruptible Power Supply) to protect sensitive imaging components. |
| 2. Are spare parts for 3D CBCT scanners readily available, and what components are most frequently replaced? | Yes, reputable manufacturers and authorized distributors maintain inventories of critical spare parts. Commonly replaced components include X-ray tubes (typically lasting 3–5 years with regular use), detector panels, gantry motors, and control board modules. In 2026, OEMs are increasingly offering modular designs to simplify part replacement. Ensure your supplier provides a documented spare parts availability policy, with minimum 7-year post-warranty support. Distributors should confirm local warehouse stock levels before finalizing procurement. |
| 3. What does the installation process for a 3D CBCT scanner involve, and is professional site preparation required? | Installation of a 3D CBCT scanner requires certified biomedical engineers or manufacturer-trained technicians. The process includes site evaluation (floor load capacity, room dimensions, radiation shielding compliance), electrical setup, network integration, and calibration. In 2026, many systems support AI-assisted calibration and remote commissioning. A lead-lined room or protective barrier is mandatory. We recommend scheduling a pre-installation site audit with the supplier to avoid delays. Average installation time ranges from 1 to 3 business days depending on room readiness. |
| 4. What warranty coverage is standard for 3D CBCT scanners in 2026, and what does it include? | As of 2026, the industry standard is a 2-year comprehensive warranty covering parts, labor, and on-site service for mechanical and electronic failures. Premium models may offer extended 3- or 5-year warranties with optional coverage for the X-ray tube and detector. Warranties typically exclude damage from power surges, improper handling, or unauthorized modifications. Always request a detailed warranty document specifying response time (e.g., 48-hour service SLA), loaner unit availability, and software update inclusion. Distributors should confirm global warranty enforceability. |
| 5. Can I purchase an extended service contract after the warranty expires, and what should it cover? | Yes, extended service contracts (ESCs) are available and highly recommended. In 2026, ESCs typically include preventive maintenance (biannual calibration and system checks), priority technical support, remote diagnostics, software updates, and coverage for high-cost components like the detector and X-ray tube. Pricing varies based on scanner model and service level (e.g., 4-hour vs. next-business-day response). We advise clinics to budget for ESCs as part of total cost of ownership—averaging 8–12% of the scanner’s purchase price annually. |
Need a Quote for 3D Cbct Scan Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


