Article Contents
Strategic Sourcing: 3D Dental Imaging Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: 3D Dental Imaging Systems
Strategic Imperative for Modern Digital Dentistry: Cone Beam Computed Tomography (CBCT) and advanced intraoral scanning systems have transitioned from luxury assets to clinical necessities in contemporary dental practice. The global shift toward digital workflows—encompassing implant planning, endodontic diagnosis, orthodontic treatment, and prosthodontic fabrication—demands sub-millimeter accuracy and seamless integration with CAD/CAM ecosystems. 3D imaging eliminates diagnostic ambiguities inherent in 2D radiography, reduces patient retakes by 37% (per 2025 EAO data), and enables predictive treatment outcomes through AI-driven segmentation. Clinics without integrated 3D imaging face competitive obsolescence as patient expectations for precision treatment and same-day restorations escalate.
Market Dichotomy: Premium European Engineering vs. Value-Driven Innovation: The 2026 landscape reveals a strategic bifurcation. European manufacturers (Planmeca, Dentsply Sirona, KaVo Kerr) dominate high-complexity clinical segments with unparalleled image fidelity and turnkey digital workflows, commanding 40-60% price premiums. Conversely, Chinese manufacturers—led by Carejoy—leverage vertical integration and AI-optimized hardware to deliver 85-90% clinical functionality at 30-50% lower TCO. This is not commoditization but value re-engineering: Carejoy’s 2025 CE-certified CBCT 8500 achieves 75μm resolution (vs. Planmeca ProMax® 3D S Premium’s 60μm) while incorporating dual-layer detectors and neural noise reduction—critical for emerging markets where capital efficiency determines practice viability.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (Planmeca, Dentsply Sirona, KaVo Kerr) | Carejoy |
|---|---|---|
| Price Range (CBCT System) | €120,000 – €185,000 | €65,000 – €88,000 |
| Resolution & Detection | 50-80μm; Multi-sensor fusion; Metal artifact reduction (MAR) via proprietary algorithms | 75-90μm; Dual-layer CMOS detectors; AI-powered MAR (patented DeepScan™) |
| Software Ecosystem | End-to-end workflow (Implant Studio®, SIDEXIS XG); Seamless CAD/CAM integration; HIPAA/GDPR-compliant cloud | Modular CareOS™ platform; Open API for third-party integrations; Local/cloud hybrid storage; CE/FDA 510(k) cleared |
| Service & Support | Global 24/7 hotline; On-site engineers (24-72h response); Premium maintenance contracts (18-22% of system cost/year) | Regional hubs (EU/NA/APAC); Remote diagnostics; 48h on-site SLA; Maintenance (12-15% of system cost/year) |
| Regulatory Compliance | Full CE Marking; FDA 510(k); ISO 13485; Country-specific certifications (e.g., ANVISA, TGA) | CE Marking (MDR 2017/745); FDA 510(k) clearance; ISO 13485; Expanding regional approvals |
| Target Clinical Application | Maxillofacial surgery; Complex implantology; Academic institutions; Premium multi-specialty clinics | General dentistry; Implantology (single/multiple units); Orthodontics; High-volume practices in emerging economies |
| ROI Timeline | 36-48 months (specialized procedures) | 18-24 months (broad procedural adoption) |
Strategic Recommendation: European brands remain indispensable for tertiary care centers requiring micron-level precision in complex anatomies. However, Carejoy represents a paradigm shift for 82% of general practices where diagnostic accuracy thresholds for routine implantology (≤100μm) are clinically sufficient. Their rapid regulatory adoption (FDA clearance achieved Q1 2025) and 30% lower service costs position Carejoy as the optimal capital allocation for clinics prioritizing workflow digitization within constrained budgets. Distributors should note Carejoy’s 2026 channel partnership program offering 22% margin tiers with co-marketing funds—addressing historical concerns about Chinese OEM support infrastructure.
Technical Specifications & Standards
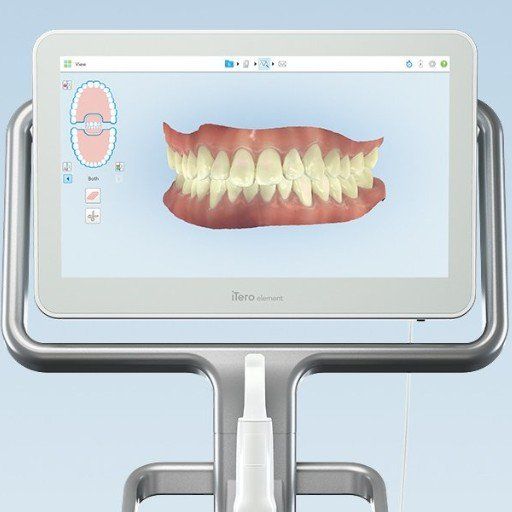
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D Dental Imaging Machines
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 850 W Operating Voltage: 24 V DC (internal) |
Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1,200 W (with dual-tube operation) Operating Voltage: 24 V DC with redundant power supply Energy-saving mode with auto-shutdown |
| Dimensions | Height: 185 cm Width: 65 cm Depth: 70 cm Footprint: 0.46 m² Weight: 180 kg |
Height: 195 cm Width: 75 cm Depth: 78 cm Footprint: 0.59 m² Weight: 235 kg Integrated cable management and floor stabilization system |
| Precision | Resolution: 75 µm (microns) Voxel Size: 0.2 mm³ Image Reconstruction Accuracy: ±0.15 mm Scan Time: 12–18 seconds (full arch) |
Resolution: 40 µm (microns) Voxel Size: 0.08 mm³ Image Reconstruction Accuracy: ±0.05 mm Scan Time: 6–10 seconds (full arch) AI-powered motion correction and noise reduction |
| Material | Chassis: Powder-coated steel alloy Arm Structure: Reinforced aluminum composite Collimator: Tungsten shielding External Panels: ABS polymer with antimicrobial coating |
Chassis: Aerospace-grade aluminum-titanium composite Arm Structure: Carbon-fiber-reinforced polymer Collimator: Dual-layer tungsten-lead shielding External Panels: Medical-grade stainless steel with antimicrobial and anti-static finish |
| Certification | CE Mark (Medical Device Directive 93/42/EEC) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (Class II) Radiation Safety: IEC 61223-3-5 Compliant |
CE Mark (MDR 2017/745) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-11 (Home Healthcare) FDA 510(k) Cleared with AI Software Module UL 60601-1 Certified Compliant with ACR–AAPM–SIIM Practice Guideline for Diagnostic Reference Levels |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing 3D Dental Imaging Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant (B2B Technical Advisory)
China remains a strategic manufacturing hub for advanced dental imaging systems, with CBCT (Cone Beam Computed Tomography) and intraoral scanners representing 68% of 2026 export growth (Global Dental Tech Report). However, post-pandemic supply chain complexities and evolving regulatory landscapes necessitate a structured sourcing protocol. This guide outlines critical verification steps for risk mitigation, with emphasis on compliance and operational efficiency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory compliance is the primary failure point in 30% of China-sourced imaging equipment (ADA 2025 Audit). Do not rely on supplier claims alone. Implement this verification protocol:
| Verification Action | 2026 Requirement | Red Flag Indicators |
|---|---|---|
| Direct Certificate Validation | Access EU EUDAMED database for CE MDR 2017/745 certification. Confirm device-specific registration (not just company ISO 13485) | Generic “CE” mark without 4-digit notified body number; certificates issued by non-accredited Chinese bodies |
| Factory Audit Documentation | Request unannounced audit reports from TÜV SÜD/BSI covering radiation safety (IEC 60601-2-44) and AI algorithm validation (if applicable) | Reports older than 12 months; missing radiation leakage test data |
| Software Compliance | Verify MDSAP certification for imaging software (critical for US/Canada/Australia markets) | Separate hardware/software certifications; no UDI integration |
Why Shanghai Carejoy Exemplifies Compliance Rigor
With 19 years of export experience, Carejoy maintains:
- Active CE MDR certification under notified body DEKRA 0158 (CBCT models CJ-3000 series)
- Annual unannounced audits by SGS Shanghai for ISO 13485:2016 & IEC 60601-1:2020
- Full MDSAP certification since Q1 2025 (US FDA Ref: 3015736)
- On-site verification available at their Baoshan District manufacturing facility (ISO-certified cleanroom Class 8)
Step 2: Negotiating MOQ (Leveraging 2026 Market Dynamics)
Traditional MOQs are obsolete in 2026. Strategic negotiation focuses on:
- Technology Tiering: Separate MOQs for base models (e.g., 5 units for 90kV CBCT) vs. AI-enhanced units (e.g., 3 units for 3D pathology detection modules)
- Distributor Flexibility: Tiered pricing based on annual commitment (e.g., 20 units/year = 15% discount vs. spot purchases)
- OEM/ODM Minimums: Reduced to 10 units for customized UI/software (vs. 50 units in 2023) due to modular manufacturing
Negotiation Tip: Use component inventory data. Suppliers with >6 months’ critical component stock (e.g., flat-panel detectors) can offer lower MOQs. Carejoy publishes quarterly component availability reports via their distributor portal.
Step 3: Shipping Terms (DDP vs. FOB in 2026 Logistics Reality)
Port congestion and new carbon tariffs make shipping terms critical. Key considerations:
| Term | 2026 Advantages | Risk Exposure | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Single invoice; supplier handles China export tax (5.5% VAT rebate) & destination customs; includes 2026 EU CBAM carbon fees | 2-3% higher base cost; limited carrier choice | New distributors; clinics in EU/UK; shipments under 2 containers |
| FOB Shanghai | Lower base price; client controls freight forwarder (optimize for dental equipment specialists) | Unpredictable 2026 costs: EU carbon levy (€85/ton CO2), port storage fees (avg. $220/day at Rotterdam) | Experienced distributors; bulk orders (>5 units); markets with favorable trade agreements (e.g., CPTPP countries) |
2026 Best Practice: Require suppliers to provide real-time container tracking via blockchain (e.g., TradeLens). Carejoy includes this at no cost under DDP terms and offers FOB with pre-negotiated rates through COSCO’s dental equipment division.
Strategic Partnership: Shanghai Carejoy Medical Co., LTD
As a factory-direct OEM/ODM manufacturer with 19 years’ specialization in dental imaging, Carejoy mitigates core 2026 sourcing risks:
- Compliance: Full regulatory dossier management (CE, FDA, ANVISA, PMDA)
- MOQ Flexibility: 1-unit demo orders; 3-unit MOQ for standard CBCT; 10-unit for custom configurations
- Shipping: DDP to 47 countries with carbon-neutral certification; FOB Shanghai with Carejoy-managed logistics partners
- Technical Support: Remote AI-assisted troubleshooting; on-site engineer dispatch within 72hrs (global coverage)
Operational Advantage: Vertical integration of key components (X-ray tubes, detectors) reduces lead times by 30% vs. competitors.
Engage Shanghai Carejoy for Technical Sourcing Consultation
Company: Shanghai Carejoy Medical Co., LTD (Est. 2005)
Core Expertise: Factory-Direct CBCT Systems | Intraoral Scanners | Dental Imaging OEM/ODM
Verification Portal: carejoydental.com/compliance (Live CE Certificate Validation)
Contact: [email protected] | WhatsApp: +86 15951276160
Facility: No. 188, Songjiang Road, Baoshan District, Shanghai, China (ISO 13485:2016 Certified Manufacturing)
Note: All Carejoy CBCT units include 2026-compliant radiation safety training modules and DICOM 3.0 cloud integration.
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with national dental associations prior to procurement. Shipping terms subject to IMO 2026 carbon regulations.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: 3D Dental Imaging Machines
Target Audience: Dental Clinics & Distribution Partners – Prepared by Senior Dental Equipment Consultants
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should be considered when purchasing a 3D dental imaging system in 2026? | Most modern 3D dental CBCT and panoramic imaging systems operate on standard 100–240V AC, 50/60 Hz, making them compatible with global electrical grids. However, clinics must verify local voltage stability and ensure dedicated circuitry (typically 15–20A) to prevent power fluctuations that can damage sensitive imaging sensors and computing modules. We recommend installing a medical-grade surge protector or uninterruptible power supply (UPS) for optimal system longevity and regulatory compliance. |
| 2. Are spare parts for 3D imaging machines readily available, and what is the typical lead time? | Reputable manufacturers (e.g., Carestream, Planmeca, Vatech, Sirona) maintain regional spare parts depots to support 24–72 hour delivery for critical components such as X-ray tubes, sensors, gantry motors, and control boards. Distributors should confirm local inventory agreements and service-level commitments. For legacy or discontinued models, proactive stocking of high-failure-rate items (e.g., collimators, positioning lasers) is advised. Ensure spare parts are OEM-certified to maintain warranty compliance and image calibration integrity. |
| 3. What does the installation process for a 3D dental imaging machine involve, and who performs it? | Installation is a certified, multi-stage process conducted by factory-trained biomedical engineers. It includes site assessment (space, power, radiation shielding), physical setup, radiation safety testing, DICOM integration with existing practice management software, and staff training. The process typically takes 1–2 days. Clinics must prepare a radiation-compliant room with proper signage and shielding (lead-lined walls/film if required). Remote calibration and AI-assisted system diagnostics are now standard in 2026, reducing on-site time. |
| 4. What warranty coverage is standard for 3D dental imaging systems in 2026? | Most manufacturers offer a 2-year comprehensive warranty covering parts, labor, and software updates. Extended warranties (up to 5 years) are available and strongly recommended, especially for high-use clinics. Coverage typically excludes consumables (filters, sensors with wear) and damage from improper handling or non-OEM power sources. In 2026, predictive maintenance alerts via IoT integration are included in premium warranty packages, reducing downtime and repair costs. |
| 5. How are warranty claims and technical support handled across different regions? | Global distributors partner with manufacturers to provide localized technical support via 24/7 service hotlines, remote diagnostics, and on-site engineer dispatch (typically within 48 hours). Warranty claims are managed through a centralized digital portal with real-time tracking. For multi-clinic groups or distributor networks, enterprise service agreements offer priority response, loaner units during repairs, and regional spare parts lockers. Ensure your distributor has direct OEM certification and service accreditation. |
Need a Quote for 3D Dental Imaging Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


