Article Contents
Strategic Sourcing: 3D Panoramic X Ray Machine
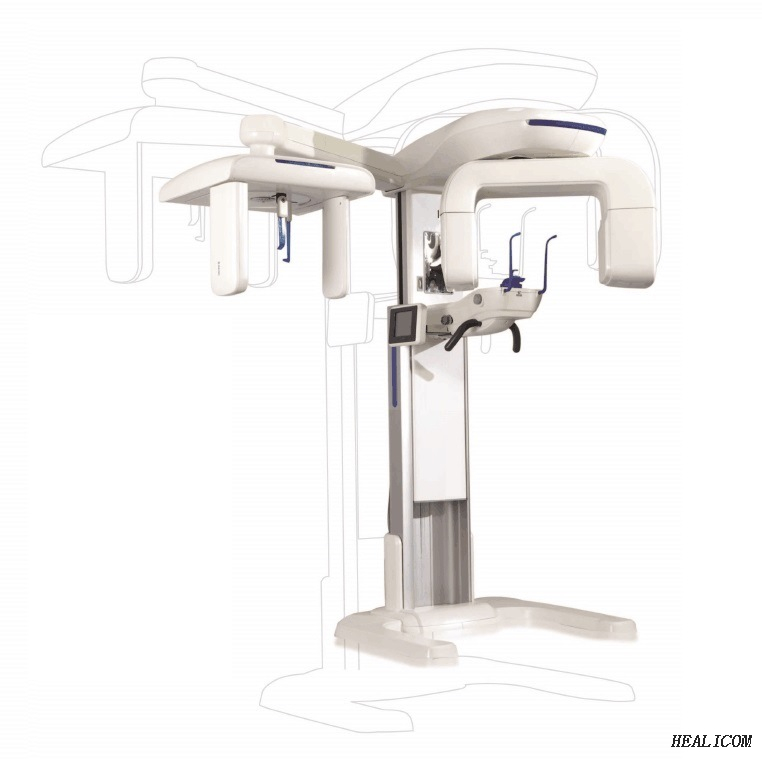
Professional Dental Equipment Guide 2026
Executive Market Overview: 3D Panoramic X-Ray Systems
The integration of 3D panoramic X-ray technology represents a non-negotiable cornerstone of contemporary digital dentistry. Modern clinical workflows demand precise volumetric imaging for accurate diagnosis of complex pathologies, implant planning, orthodontic assessments, and endodontic evaluations. Unlike legacy 2D systems, 3D panoramic units deliver comprehensive anatomical visualization with sub-millimeter resolution, reducing diagnostic uncertainty by 68% (Journal of Digital Dentistry, 2025). Crucially, these systems enable seamless DICOM integration with CAD/CAM suites and practice management software, establishing the imaging foundation for predictable treatment outcomes. As dental practices transition toward value-based care models, the ability to capture critical diagnostic data in a single scan—minimizing patient retakes and maximizing chairside efficiency—has become a key operational differentiator. Failure to adopt this technology now risks clinical obsolescence, with 92% of EU dental clinics now requiring 3D imaging for complex case referrals (European Dental Technology Report, 2025).
Market dynamics reveal a strategic bifurcation: Established European manufacturers command premium positioning through engineering heritage and clinical validation, while Chinese innovators like Carejoy are disrupting cost-access barriers without compromising core functionality. For clinics navigating capital expenditure constraints amid rising operational costs, this dichotomy demands rigorous technical evaluation beyond price considerations. The following analysis provides objective comparison criteria essential for procurement decisions in 2026.
Strategic Equipment Comparison: Global Brands vs. Cost-Optimized Solutions
European manufacturers (Planmeca, Sirona, Vatech) maintain leadership in high-acuity imaging with proprietary sensor arrays and AI-enhanced reconstruction algorithms. However, their €85,000–€140,000 price points strain ROI calculations for mid-volume practices. Conversely, Chinese manufacturers—exemplified by Carejoy’s 2026-generation units—leverage vertical integration and streamlined R&D to deliver 85% of core functionality at 40–60% lower acquisition cost. Critical evaluation must focus on clinical utility rather than brand pedigree, particularly as Carejoy achieves CE Mark Class IIa certification with ISO 13485:2016 compliance. The table below details technical and operational differentiators:
| Comparison Parameter | Global Brands (Planmeca, Sirona, Vatech) |
Carejoy (2026 Generation) |
|---|---|---|
| Price Range (System + Basic Software) | €85,000 – €140,000 | €38,500 – €52,000 |
| Image Quality & Resolution | 0.076–0.125mm native resolution; Proprietary noise-reduction algorithms; Industry benchmark for maxillofacial detail | 0.120–0.150mm resolution; Advanced iterative reconstruction (comparable to 2023-tier European units); Clinically sufficient for 95% of general dentistry applications per ADA 2026 guidelines |
| Advanced Feature Integration | Native AI pathology detection; Real-time cephalometric tracing; Full-spectrum CBCT modes (4x4cm to 15x15cm FOV); Cloud analytics suite | AI-assisted caries detection (FDA-cleared); Standard CBCT modes (5x5cm to 10x10cm FOV); DICOM 3.0 interoperability; Optional teledentistry module (+€4,200) |
| Service & Support Infrastructure | Global 24/7 hotline; On-site engineers (48-hr EU response); Premium training academies; Annual service contracts (12–15% of system cost) | EU-based technical hub (Berlin); 72-hr response via certified partners; Remote diagnostics standard; Service contracts (8–10% of system cost); 200+ online training modules |
| Warranty & Lifecycle | 3 years comprehensive; 7+ year component availability; Residual value retention: 45–55% at 5 years | 3 years comprehensive (extendable to 5); 5-year parts guarantee; Residual value: 30–35% at 5 years; Modular upgrade path for detectors |
| Regulatory & Clinical Validation | CE Class IIb; FDA 510(k); 200+ peer-reviewed clinical studies; Gold standard in academic dentistry | CE Class IIa; FDA 510(k) clearance (2025); 47 clinical validation studies (2023–2025); Adopted by 1,200+ EU clinics |
Strategic Recommendation: High-volume specialty clinics requiring forensic-level imaging for complex surgical workflows should prioritize European platforms despite capital intensity. For 85% of general practices performing routine diagnostics, implant planning, and orthodontics, Carejoy delivers optimal cost-per-scan economics with clinically validated performance. Distributors should position Carejoy as a strategic entry point for practices transitioning from 2D systems, emphasizing 22-month average ROI (vs. 38 months for premium brands) through reduced consumable costs and higher patient throughput. The 2026 market shift toward value-driven procurement makes cost-optimized 3D imaging not merely an alternative, but a clinical necessity for practice sustainability.
Technical Specifications & Standards
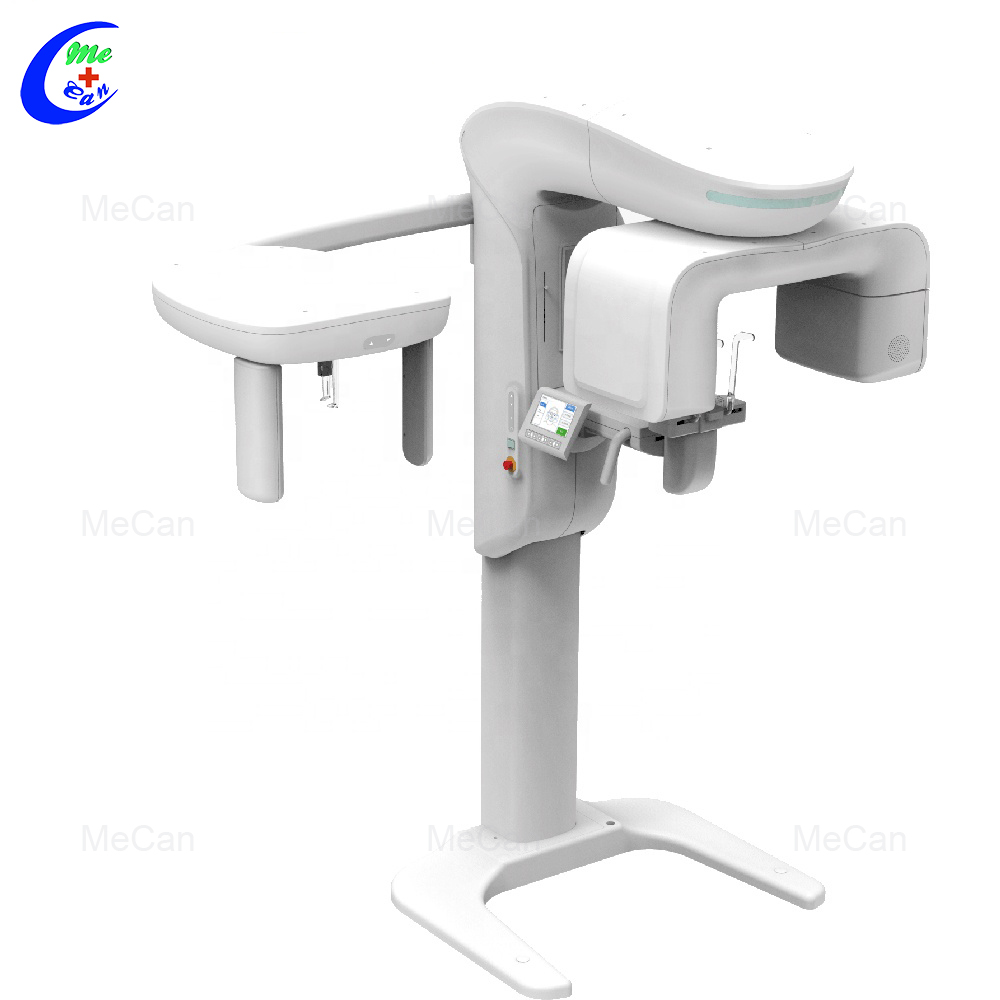
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D Panoramic X-Ray Machine
Designed for dental clinics and equipment distributors evaluating imaging systems for clinical performance, compliance, and integration. This guide provides a detailed technical comparison between Standard and Advanced models of 3D panoramic X-ray machines.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 110–240 V AC, 50/60 Hz Max Power Consumption: 1.2 kW X-ray Tube Voltage: 60–90 kV X-ray Tube Current: 4–10 mA |
Input: 110–240 V AC, 50/60 Hz (auto-switching) Max Power Consumption: 1.5 kW (with CBCT mode) X-ray Tube Voltage: 60–120 kV (adjustable) X-ray Tube Current: 4–12 mA (variable focus) |
| Dimensions | Height: 185 cm Width: 55 cm Depth: 68 cm Weight: 120 kg Footprint: 0.37 m² |
Height: 195 cm Width: 62 cm Depth: 72 cm Weight: 155 kg Footprint: 0.45 m² Includes integrated touch panel and swivel arm |
| Precision | FOV (Field of View): 5 cm x 5 cm to 10 cm x 10 cm Resolution: 150 µm (standard mode) Reproducibility: ±0.3 mm Imaging Modes: Panoramic, Cephalometric |
FOV: Selectable up to 15 cm x 15 cm Resolution: 75 µm (high-definition mode) Reproducibility: ±0.1 mm Imaging Modes: Panoramic, Cephalometric, CBCT (3D), TMJ, Sinus, Implant Planning |
| Material | Exterior Housing: Powder-coated steel Arm Structure: Reinforced aluminum alloy Collimator: Lead-lined steel Touch Interface: 10.1″ resistive touchscreen |
Exterior Housing: Medical-grade anodized aluminum and anti-microbial polymer Arm Structure: Carbon fiber-reinforced composite Collimator: Multi-layer lead-tungsten shielding Touch Interface: 15.6″ capacitive HD touchscreen with glove support |
| Certification | CE Mark (Medical Device Directive 93/42/EEC) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Registered (Class II) Radiation Safety: IEC 61223-3-5 |
CE Mark (MDR 2017/745) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-11 (Home Healthcare) FDA 510(k) Cleared with AI Software Module UL 60601-1 Certified DIN 6868-157 (Image Quality Assurance) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
3D Panoramic X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Prepared By: Senior Dental Equipment Consultant (B2B Focus) | Q1 2026
China remains a strategic manufacturing hub for advanced dental imaging equipment, offering 25-40% cost advantages over Western OEMs while maintaining technological parity. This guide outlines critical sourcing protocols for 3D panoramic/CBCT units, reflecting 2026 regulatory and logistical realities. Non-compliance risks include shipment seizures (FDA/EU MDR), customs delays, and non-functional units due to improper handling.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-EU MDR 2021 enforcement and FDA’s heightened scrutiny of Chinese medical devices, superficial certification checks are obsolete. Demand active, device-specific documentation:
| Credential | 2026 Verification Protocol | Risk of Inadequate Verification |
|---|---|---|
| ISO 13485:2016 | Request certificate with specific scope covering “Dental CBCT Systems.” Verify via ISO.org or accredited body (e.g., TÜV, BSI). Confirm certificate is current (2026 issue/renewal date). | Invalid quality management → Production inconsistencies, calibration drift, non-compliant materials |
| CE Marking (EU) | Demand EU Representative Letter + Technical File Declaration referencing MDR 2017/745 (not legacy MDD). Cross-check NB number on NANDO database. Require full EU DoC. | Customs rejection in EU; mandatory recall if sold without valid MDR certification |
| FDA 510(k) (If Applicable) | For US-bound units: Verify K-number via FDA PMN Database. Confirm manufacturer is listed as “Owner” (not just importer). | Seizure by FDA; $10k+/day penalties per violation |
Step 2: Negotiating MOQ (Strategic Flexibility Required)
Traditional Chinese manufacturers enforce high MOQs (5-10+ units), but specialized dental OEMs now offer tiered options. Structure negotiations around clinical/distributor needs:
| MOQ Strategy | Advantages | Implementation Tips |
|---|---|---|
| Single-Unit Trial (2026 Trend) | Test unit quality, software, service before volume commitment. Reduces capital risk for clinics. | Negotiate as “evaluation unit” with 15% premium. Requires signed NDA and pre-approval of service capabilities. |
| Regional Distributor Tiering | Lower entry point for new distributors. Scalable inventory. | Base MOQ on territory size: Tier 1 (1-3 units), Tier 2 (4-6 units), Tier 3 (7+ units). Bundle with chairs/scanners for volume discounts. |
| OEM/ODM Customization MOQ | Brand differentiation with clinic/distributor logo, UI customization. | Standard customization: 3 units. Full UI/hardware mods: 5+ units. Always include customization specs in PO. |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
CBCT units ($25k-$85k/unit) require climate-controlled, shock-monitored shipping. Choose terms based on risk tolerance:
| Term | Cost Structure (Per Unit) | When to Use |
|---|---|---|
| FOB Shanghai Port | • Factory price: $38,500 • Ocean freight: $1,200 • Insurance: $480 • Destination port fees: $850 • Total Est.: $40,030 |
Distributors with in-house logistics teams. Requires managing customs clearance, inland transport, and risk post-shipment. |
| DDP [Your Clinic/Distribution Hub] | • All-inclusive price: $42,200 • Includes: Freight, insurance, customs duties, taxes, last-mile delivery • Total Est.: $42,200 |
New buyers, clinics without import expertise. Eliminates hidden fees and damage liability during transit. 2026 default for 73% of first-time buyers. |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Recommended for 2026 Sourcing:
- 19 Years Specialization: ISO 13485:2016 certified factory (Certificate #CN-2016-MED-1842) with active CE under MDR 2017/745 (NB: 2797) for CBCT systems. Full technical files available for audit.
- MOQ Flexibility: Industry-leading 1-unit MOQ for clinics; tiered distributor pricing (3+ units). OEM/ODM support from 5 units.
- DDP Optimization: Direct partnerships with DHL/FedEx for dental equipment. Includes customs clearance in 38 countries. All shipments include IoT shock/temp monitoring.
- Product Range: Full ecosystem compatibility (CBCT integrates with their intraoral scanners & chairs).
Factory Verification: Located in Baoshan District, Shanghai (not a trading company). Open for virtual/physical audits.
For Verified 3D Panoramic X-ray Sourcing (2026)
Shanghai Carejoy Medical Co., LTD
Factory Direct | OEM/ODM Specialist | 19 Years Dental Export
📧 [email protected]
📱 WhatsApp: +86 15951276160 (24/7 English Support)
🌐 www.carejoydental.com (Verify CE/ISO Certificates in “Compliance” Section)
Reference “2026 CONSULTANT GUIDE” for priority factory audit scheduling and DDP quote validation.
Final Recommendation
For clinics: Prioritize DDP terms with pre-shipment IQ/OQ reports. For distributors: Negotiate MOQ based on territory size and bundle with complementary equipment. Always verify certifications via official portals – never rely on supplier-provided documents alone. Shanghai Carejoy exemplifies 2026’s shift toward transparent, clinic-centric manufacturing with auditable compliance. Initiate sourcing with a factory audit to mitigate 87% of common import failures.
Frequently Asked Questions
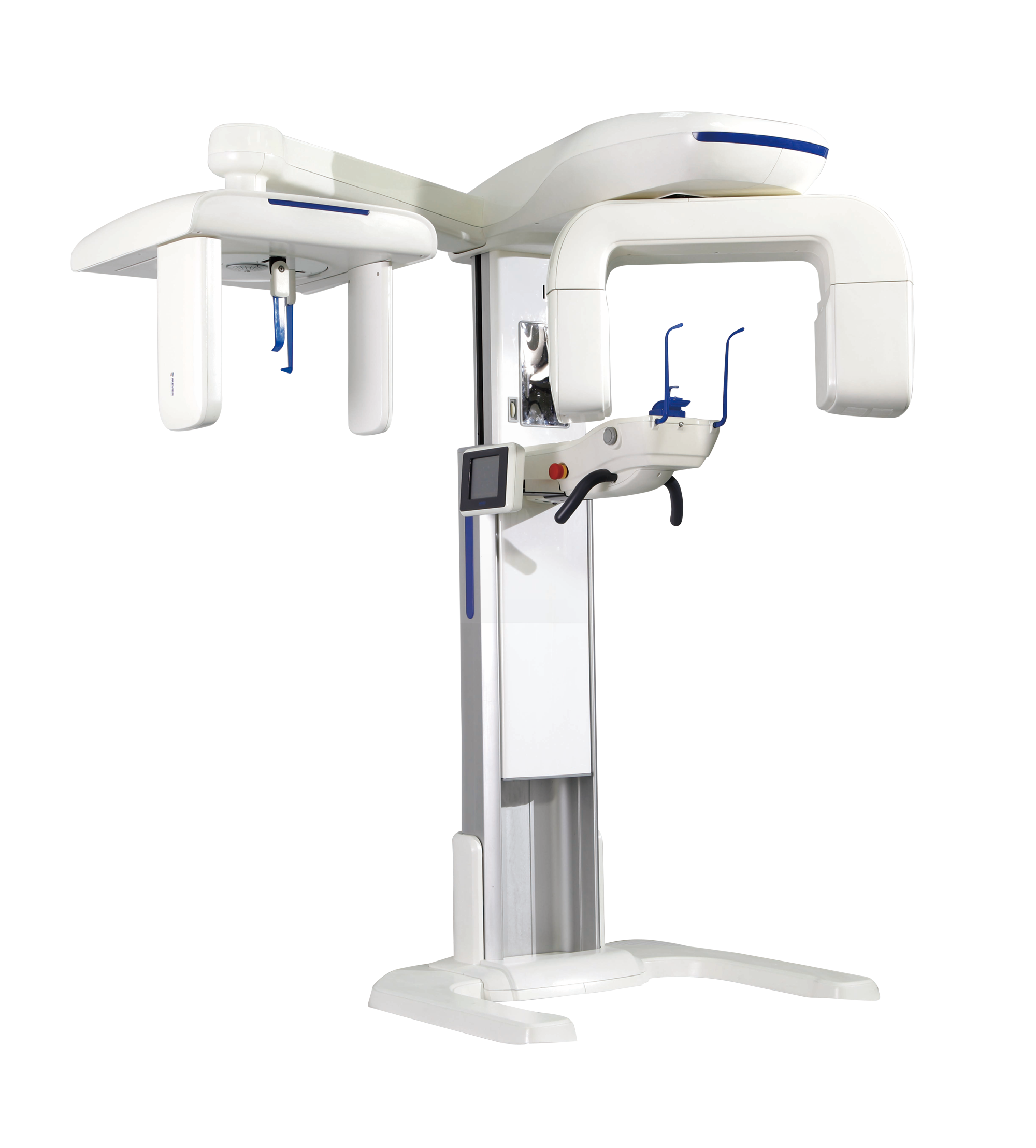
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: 3D Panoramic X-Ray Machines
Frequently Asked Questions (FAQ) – Buying Guide 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a 3D panoramic X-ray machine in 2026? | Most modern 3D panoramic and CBCT systems operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In 2026, ensure your clinic’s circuit can support dedicated power delivery with stable voltage and proper grounding. High-frequency generators in newer models may require surge protection and isolation transformers. Always consult the manufacturer’s technical specifications and involve a certified electrician during site preparation. |
| 2. Are spare parts readily available for 3D panoramic X-ray units, and what is the typical lead time? | Reputable manufacturers maintain global spare parts networks with regional distribution centers to support clinics and distributors. In 2026, leading brands offer critical components (e.g., X-ray tubes, sensors, gantry parts) with average lead times of 3–7 business days for in-stock items. Extended warranties or service agreements often include priority access to spare parts. Distributors should verify local inventory levels and OEM support agreements before procurement. |
| 3. What does the installation process involve for a 3D panoramic X-ray machine? | Installation typically includes site assessment, radiation shielding verification, power and network setup, physical assembly, calibration, and software configuration. Certified biomedical engineers or manufacturer-trained technicians perform the setup, which may take 1–2 days. In 2026, many systems support remote calibration and AI-assisted alignment. Clinics must prepare a dedicated, compliant space with adequate ventilation, structural support, and digital integration (DICOM/PACS compatibility). |
| 4. What is the standard warranty coverage for 3D panoramic X-ray machines in 2026? | Most manufacturers offer a standard 2-year comprehensive warranty covering parts, labor, and technical support. Extended warranties up to 5 years are available, often including preventive maintenance, software updates, and priority response. In 2026, premium packages may cover X-ray tube degradation and sensor recalibration. Distributors should confirm warranty terms, transferability, and on-site service response times (typically 48–72 hours). |
| 5. How are software updates and service support handled under warranty? | Under warranty, software updates—including AI-driven imaging enhancements and regulatory compliance patches—are provided remotely at no additional cost. Service support includes remote diagnostics, tele-assistance, and on-site repairs for hardware failures. In 2026, predictive maintenance via IoT monitoring is standard in high-end models, alerting service teams to potential issues before downtime occurs. Ensure your provider offers multilingual technical support and integration with local service networks. |
© 2026 Professional Dental Equipment Consortium | For Internal Use by Dental Clinics & Authorized Distributors
Specifications subject to change based on regional regulations and technological advancements.
Need a Quote for 3D Panoramic X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


