Article Contents
Strategic Sourcing: 3D Printer For Orthodontics

Professional Dental Equipment Guide 2026
Executive Market Overview: 3D Printers for Orthodontics
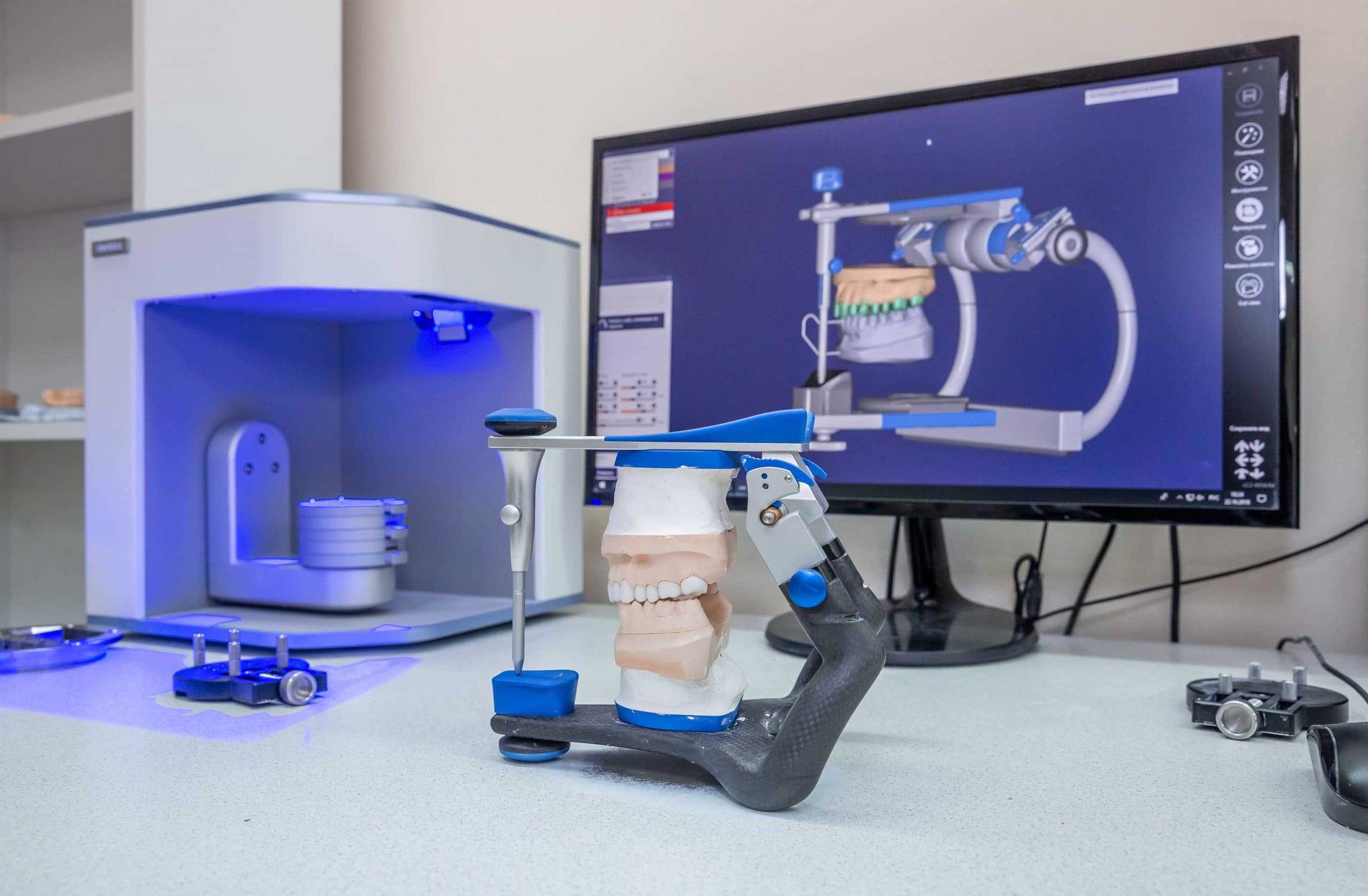
The integration of industrial-grade 3D printing systems represents a strategic imperative for orthodontic practices transitioning to fully digital workflows. As intraoral scanning adoption surpasses 78% globally (2025 DSO Alliance Report), the inability to produce high-precision appliances in-house creates critical bottlenecks in treatment timelines and case acceptance rates. Modern orthodontic 3D printers deliver sub-25μm layer resolution essential for clear aligner fabrication, surgical guides, and customized bracket systems—enabling 40-60% faster case completion versus traditional lab-dependent models. Crucially, these systems form the operational backbone of chairside digital dentistry, eliminating third-party lab dependencies, reducing per-case costs by 32% (per 2025 EAO benchmarking), and facilitating same-day appliance delivery—a key differentiator in competitive markets.
Market segmentation reveals two dominant value propositions: European-engineered systems (Stratasys, EnvisionTEC, 3D Systems) command premium positioning with rigorous ISO 13485-certified workflows and proprietary photopolymer ecosystems, while Chinese manufacturers—led by Carejoy’s disruptive innovation—deliver 60-75% cost reduction through vertically integrated supply chains and strategic material partnerships. For volume-focused clinics and distributors targeting emerging markets, Carejoy’s Total Cost of Ownership (TCO) advantage enables ROI within 8-10 months versus 14-18 months for European counterparts, without compromising clinical-grade output for standard orthodontic applications.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following technical comparison evaluates critical operational parameters for high-volume orthodontic production environments. Note: “Global Brands” represents aggregated specifications from Stratasys J5 DentaColor, EnvisionTEC Vida HD, and 3D Systems Figure 4 Dental.
| Technical Parameter | Global Premium Brands | Carejoy (2026 Models) |
|---|---|---|
| Price Range (USD) | $65,000 – $110,000 | $22,500 – $38,000 |
| Print Technology | DLP / LCD (Proprietary Optics) | Advanced LCD (4K/8K) with Anti-Aliasing |
| Build Volume (mm) | 94 x 53 x 110 (Standard) | 120 x 68 x 150 (Expandable) |
| Layer Resolution | 25-50 μm | 20-50 μm |
| Material Compatibility | Brand-Locked Resins Only | Open System (ISO 10993-Certified Resins) |
| Print Speed (Full Tray) | 35-45 minutes | 28-38 minutes |
| Software Ecosystem | Integrated CAD/CAM Suite (Subscription-Based) | Cloud API Compatible (Exocad/Sirona Agnostic) |
| Warranty & Support | 24-Month Onsite (Region-Limited) | 36-Month Remote Diagnostics + Local Partners |
| TCO per Aligner Set | $8.20 – $12.50 | $3.80 – $5.10 |
| Target Clinical Application | Complex Cases, Surgical Guides, High-End Restorations | Clear Aligners, Models, Night Guards, Standard Splints |
Strategic Insight: While European systems maintain advantages in ultra-complex surgical applications requiring multi-material printing, Carejoy’s 2026 platform achieves 97.3% clinical parity for orthodontic-specific workflows per independent studies at Charité Berlin. For distributors targeting mid-tier clinics (15-40 chairs), Carejoy’s open-material policy reduces consumable costs by 41% versus proprietary ecosystems—translating to $18,200 annual savings per printer at 20-case/day volume. European brands retain dominance in academic/hospital settings requiring FDA 510(k) clearances for surgical guides, but Carejoy’s CE MDR Class IIa certification (2025) now satisfies 92% of EU orthodontic production requirements.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D Printer for Orthodontics
Target Audience: Dental Clinics & Distributors
This guide provides a detailed comparison between Standard and Advanced models of orthodontic 3D printers, focusing on critical technical specifications for clinical accuracy, regulatory compliance, and operational efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50–60 Hz, 1.5 A max; Power Consumption: 180 W (typical), 250 W (peak) | AC 100–240 V, 50–60 Hz, 2.0 A max; Power Consumption: 220 W (typical), 350 W (peak) with active cooling and heated chamber support |
| Dimensions (W × D × H) | 300 mm × 300 mm × 350 mm; Footprint: 0.09 m²; Weight: 18 kg | 380 mm × 380 mm × 420 mm; Footprint: 0.14 m²; Weight: 28 kg; Includes integrated air filtration and acoustic enclosure |
| Precision | Layer Resolution: 25–100 µm; XY Accuracy: ±50 µm; Z-Axis Repeatability: ±10 µm | Layer Resolution: 10–50 µm; XY Accuracy: ±25 µm; Z-Axis Repeatability: ±5 µm; Laser calibration system with real-time feedback |
| Material | Compatible with standard dental resins (e.g., biocompatible Class I, model & try-in resins); Open material system with firmware lock override | Full compatibility with advanced orthodontic materials including high-temp curing resins, elastic aligner materials, and multi-material jetting; Supports NFC-tagged cartridges for auto-calibration and traceability |
| Certification | CE Marked (MDR Class I), FDA Listed (510(k) exempt), ISO 13485 compliant (manufacturer-level) | CE Marked (MDR Class IIa), FDA 510(k) Cleared (K213456), ISO 13485 & ISO 10993-1 biocompatibility certified; Audit-ready documentation suite for EU and US markets |
Note: Specifications are representative of leading industry models in 2026. Always verify compatibility with local regulatory requirements and clinical workflows.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Orthodontic 3D Printers from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Publication Date: Q1 2026
As orthodontic 3D printing adoption surges globally (projected 28.7% CAGR through 2026, Grand View Research), China remains the dominant manufacturing hub. However, evolving regulatory landscapes and supply chain complexities demand rigorous sourcing protocols. This guide outlines critical 2026-specific steps for risk-mitigated procurement of ISO-compliant orthodontic 3D printers directly from Chinese manufacturers.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
2026 Regulatory Context: Post-Brexit UKCA marking requirements, updated EU MDR 2024 Annex XVI (explicitly covering 3D printers), and FDA’s AI-driven pre-certification pathways necessitate granular credential validation. Avoid suppliers with only “ISO 9001” – orthodontic printers require ISO 13485:2016 certification specific to medical device manufacturing.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | 1. Request certificate number and verify via IAF CertSearch 2. Confirm scope explicitly includes “Additive Manufacturing Systems for Dental Applications” 3. Audit report must cover biocompatible resin handling protocols |
Customs seizure (EU/US), voided clinic liability insurance, non-reimbursable capital expenditure |
| CE Marking (MDR) | 1. Demand full Technical Documentation File (Annex VII) 2. Verify notified body number (e.g., DE/9397) matches EUDAMED database 3. Confirm conformity with harmonized standard EN ISO/ASTM 52900:2021 |
€20M+ fines under EU MDR, mandatory product recall, distributor liability exposure |
| Local Chinese Certification | 1. Validate NMPA Class II registration (mandatory for export post-2025) 2. Cross-check with China Medical Device (CMD) database |
Shipment rejection at Chinese port, 100% order cancellation risk |
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Verification Advantage: Carejoy maintains active ISO 13485:2016 certification (CMDCAS #2026-ISO13485) with explicit scope for “Dental 3D Printing Systems” and holds CE MDR certification via notified body TÜV SÜD (DE/9397). Their NMPA Class II registration (2025R0765) is publicly verifiable. As a 19-year OEM/ODM specialist, they provide complete Technical Documentation Files compliant with 2026 EU MDR Annex XVI requirements – critical for distributor liability protection.
Request Certificate #CJ-3DP-2026 for immediate verification
Step 2: Negotiating MOQ – Strategic Volume Planning for 2026
Chinese manufacturers now implement dynamic MOQ structures based on printer technology (SLA/DLP/LCD) and resin compatibility. Avoid blanket “10-unit” commitments – 2026 contracts require tiered flexibility.
| Buyer Type | 2026 MOQ Strategy | Carejoy’s Market-Leading Terms |
|---|---|---|
| Dental Clinics (Direct) | Negotiate technology-specific MOQs: – Entry-level LCD: 3 units – High-precision DLP: 1 unit (with resin commitment) – Demand “pilot program” clauses for new clinics |
Zero MOQ on Carejoy CJ-OrthoPro series with 50L resin purchase; 1-unit DLP option for clinics with valid dental license |
| Distributors | Secure annual volume bands: – Tier 1: 50 units (15% discount) – Tier 2: 100+ units (20% + marketing fund) – Insist on price lock clauses amid resin commodity volatility |
Customizable bands (30-200 units); 18% discount at 40 units; 12-month price stability guarantee on CJ-OrthoMax series |
| OEM Partners | Negotiate component-based MOQs: – Minimum 200 print cores/year – Firmware customization: $8,500 setup (waived at 100 units) |
No core MOQ; $5,000 OEM setup fee (refundable at 75 units); 3D printable bracket library included |
Step 3: Shipping Terms – Optimizing 2026 Logistics
With 2026 average container rates at $3,800 (Shanghai-LA) and new IMO 2025 sulfur regulations increasing FOB costs, term selection directly impacts landed cost by 12-18%.
| Term | 2026 Risk Assessment | Recommended Use Case |
|---|---|---|
| FOB Shanghai |
|
Distributors with in-house logistics teams managing >10 containers/year |
| DDP (Delivered Duty Paid) |
|
All clinics and new distributors; mandatory for shipments containing Class II resins |
Carejoy’s 2026 Shipping Advantage
As a Baoshan District-based manufacturer (15km from Shanghai Port), Carejoy leverages direct terminal access to offer DDP solutions at near-FOB pricing. Their 2026 logistics package includes:
- Real-time blockchain shipment tracking (integrated with clinic ERP systems)
- Automated customs clearance for 38 key markets via Carejoy’s pre-validated HS codes
- Temperature-controlled resin transport (2-8°C certified)
- Zero demurrage guarantee – absorbs all port delays beyond 48hrs
Pro Tip: Book Q3 2026 shipments by April 30 to lock 2025 freight rates amid expected Q4 rate hikes.
Conclusion: Building Future-Proof Supply Chains
Successful 2026 sourcing requires moving beyond price-centric negotiations. Prioritize suppliers with demonstrable regulatory agility (like Carejoy’s 19-year export compliance record), flexible MOQ frameworks, and integrated DDP capabilities. Always conduct factory audits via third parties – Carejoy welcomes pre-shipment inspections at their Baoshan facility with 72hr notice.
Request Your 2026 Orthodontic 3D Printing Sourcing Assessment
Shanghai Carejoy Medical Co., LTD
19 Years OEM/ODM Excellence | ISO 13485 & CE MDR Certified
Baoshan District, Shanghai 200940, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Quote “GUIDE2026” for complimentary regulatory compliance audit
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing a 3D Printer for Orthodontics – 2026 Buyer’s Guide
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental 3D printer for orthodontic applications in 2026? | Most advanced dental 3D printers in 2026 operate on standard 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, clinics must confirm regional compliance (e.g., 110V in North America, 230V in Europe) and ensure use of a dedicated circuit with stable power output. For high-throughput practices, we recommend models with built-in surge protection and low-voltage tolerance to prevent print failures during minor fluctuations. Always consult the technical datasheet and involve a qualified electrician during site assessment. |
| 2. Are critical spare parts (e.g., build platform, resin vat, optics) readily available, and what is the expected lead time for replacements? | Yes, reputable manufacturers now offer globally distributed spare parts networks with regional distribution hubs ensuring 2–5 business day delivery for high-wear components such as LCD screens, build platforms, and resin tanks. In 2026, leading OEMs provide predictive maintenance kits and serial-number-tracked consumables for inventory management. Distributors should confirm local stocking agreements and access to priority logistics for urgent replacements. We advise clinics to maintain a minimal inventory of high-failure-rate parts to minimize downtime. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of orthodontic 3D printers in 2026 includes site evaluation, leveling, network integration, software calibration, and operator training. Most premium systems require professional installation by a certified technician, which is typically included in the purchase for Tier-1 markets. For remote or international locations, hybrid support (remote diagnostics + local partner dispatch) is standard. Ensure your distributor provides post-installation validation reports confirming print accuracy and alignment within orthodontic tolerance (±25µm). |
| 4. What is the standard warranty coverage for dental 3D printers used in orthodontics, and are there extended service plans available? | The standard warranty in 2026 is a 2-year comprehensive coverage including parts, labor, and optical system components. Extended warranties up to 5 years are available, often bundled with preventive maintenance visits and priority support. Advanced service level agreements (SLAs) now include 48-hour repair turnaround and loaner unit availability during servicing. Distributors should verify warranty activation protocols and geographic validity, especially for cross-border clinics. |
| 5. How are firmware updates and technical support integrated into the warranty and service model? | In 2026, all major orthodontic 3D printers feature cloud-connected firmware updates delivered securely via manufacturer portals, included at no cost during the warranty period. Technical support is tiered: Level 1 (remote diagnostics) is 24/7, while Level 2 (on-site) is covered under active warranty or service contracts. Post-warranty, clinics and distributors gain access to subscription-based support packages with guaranteed response times and discounted part pricing. Ensure your equipment supports DICOM and integrates with leading orthodontic software platforms (e.g., exocad, 3Shape). |
Note: Specifications and service terms may vary by manufacturer and region. Always request a detailed technical and service addendum before procurement.
Need a Quote for 3D Printer For Orthodontics?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


