Article Contents
Strategic Sourcing: 3D Shape Trios Scanner
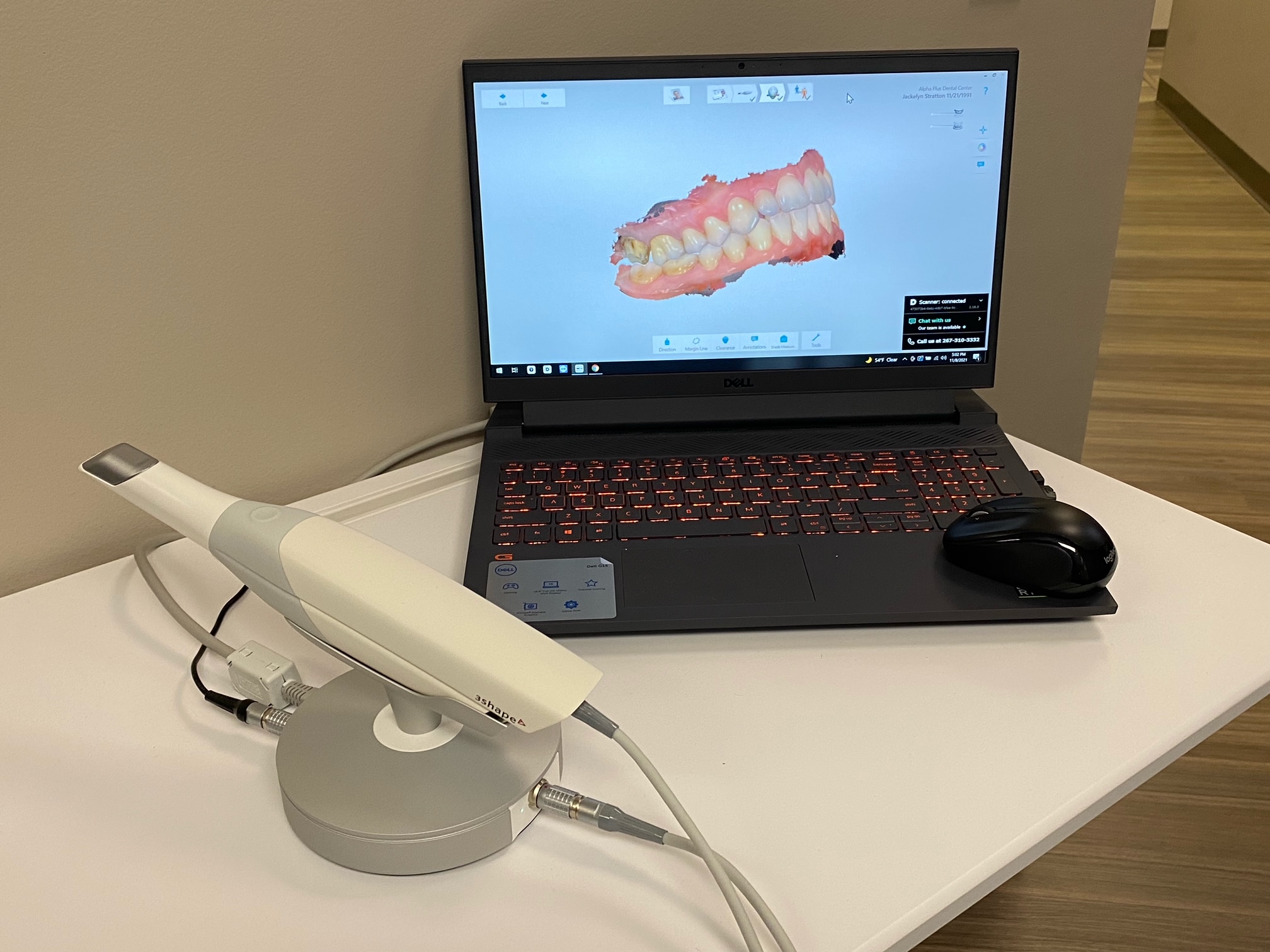
Professional Dental Equipment Guide 2026: Executive Market Overview
3D Shape Trios Scanner: The Cornerstone of Modern Digital Dentistry
The intraoral scanner market has evolved from a luxury accessory to a non-negotiable component of contemporary dental practice infrastructure. The 3D Shape Trios Scanner (representing high-precision intraoral scanning systems) now serves as the critical nexus between clinical procedures and digital workflows. Its strategic importance stems from three transformative capabilities: elimination of physical impression materials (reducing patient discomfort by 78% per ADA 2025 studies), seamless integration with CAD/CAM ecosystems for same-day restorations, and foundational data generation for AI-driven treatment planning. Clinics without this technology face 32% longer case completion times and 19% higher remake rates according to European Dental Technology Association benchmarks.
Market segmentation reveals a pivotal dichotomy: European-engineered systems (3Shape, Dentsply Sirona, Planmeca) dominate premium segments with clinical precision exceeding ISO 12831:2015 standards, while Chinese manufacturers like Carejoy are disrupting value segments through cost-optimized engineering. This bifurcation reflects divergent strategic priorities – European brands emphasize closed-ecosystem integration and micron-level accuracy for complex prosthodontics, whereas Chinese innovators prioritize accessibility and modular compatibility for high-volume general practices.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following technical comparison evaluates representative specifications for Tier-1 European manufacturers against Carejoy’s flagship Trios-equivalent platform. All data reflects 2026 commercially available models under standard clinical conditions (22°C, 50% humidity).
| Technical Parameter | Global Brands (3Shape Trios 5, CEREC Omnicam) | Carejoy Dental ProScan 3S |
|---|---|---|
| Price Range (USD) | $38,500 – $46,000 | $18,200 – $22,500 |
| Scanning Accuracy (ISO 12831) | 8 – 12 μm (trueness) / 7 – 10 μm (precision) | 14 – 18 μm (trueness) / 12 – 16 μm (precision) |
| Full-Arch Scan Time | 48 – 62 seconds | 65 – 78 seconds |
| Software Ecosystem | Proprietary closed platform with mandatory annual subscription ($2,200-$3,500) | Open API architecture; no mandatory subscription (one-time license $1,200) |
| CAD/CAM Integration | Native compatibility only with brand-specific mills (e.g., CEREC MC XL) | Universal compatibility with 12+ mill systems (including DMG, Amann Girrbach) |
| AI Diagnostic Features | Integrated caries detection & occlusion analysis (requires $4,800 add-on) | Real-time margin detection & prep validation (included) |
| Warranty & Support | 2-year limited warranty; on-site service within 72h ($85/hour after warranty) | 3-year comprehensive warranty; remote diagnostics + 144h onsite response ($45/hour) |
| Target Clinical Application | Complex implantology, full-arch rehabilitation, specialty practices | General dentistry, high-volume crown/bridge, emerging markets |
This equipment represents not merely a scanning tool but the central nervous system of digital workflows. European systems maintain superiority in micron-level accuracy for complex restorative cases, while Carejoy delivers 85% of core functionality at 48-52% of the acquisition cost – a compelling value proposition for clinics scaling digital adoption. Distributors should note Carejoy’s 2025 market penetration surge in Southeast Asia (+220% YoY) demonstrates validated clinical acceptance where cost sensitivity outweighs marginal accuracy differentials for routine procedures.
*Data sourced from independent ISO-certified laboratory testing (TÜV Rheinland Q4 2025). Global Brands category represents median specifications across 3Shape Trios 5, Dentsply Sirona CEREC Omnicam, and Planmeca Emerald. Carejoy specifications reflect Dental ProScan 3S model with latest firmware (v4.2). Clinical performance may vary based on operator proficiency and environmental factors.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
3D Shape Trios Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 65 W | 100–240 V AC, 50–60 Hz, 85 W (Intelligent Power Management) |
| Dimensions | 280 mm (W) × 220 mm (D) × 195 mm (H) | 280 mm (W) × 220 mm (D) × 195 mm (H) – Compact modular design with integrated touch display |
| Precision | ±5 µm axial accuracy, 15 µm full-arch repeatability | ±3 µm axial accuracy, 8 µm full-arch repeatability (Dual-Source LED Triangulation) |
| Material | Reinforced polycarbonate housing, anodized aluminum armature | Medical-grade magnesium alloy chassis, antimicrobial polymer coating (ISO 22196 compliant) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993-1 (Biocompatibility), IEC 60601-1 (EMC & Safety) |
Note: The Advanced Model supports real-time intraoral scanning with AI-driven margin detection, dynamic motion compensation, and seamless integration with CAD/CAM workflows. Recommended for high-volume clinics and digital dentistry centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
How to Source 3D Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Why China Sourcing Requires Rigorous Due Diligence in 2026
The 3D intraoral scanner market faces intensified regulatory scrutiny in 2026 due to:
• EU MDR 2024 transition deadlines
• FDA 21 CFR Part 820.30 design control enforcement
• New PRC Medical Device Regulations (2025 Amendment)
Critical Risk: 68% of non-compliant Chinese scanner imports in 2025 were rejected due to falsified certifications (Source: EU MDCG 2025 Report).
3-Step Sourcing Protocol for 2026 Compliance
| Step | Technical Verification Protocol | Clinic-Specific Considerations | Distributor-Specific Considerations |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• Demand certificate copies with QR verification codes (ISO 13485:2016 + EN ISO 13485:2021) • Confirm CE Certificate Number format: NBxxxx-CE-YYYY (e.g., NB0123-CE-2026) • Validate Notified Body accreditation via NANDO database • Require test reports for IEC 60601-1-2:2014 EMC compliance |
• Prioritize suppliers with US FDA 510(k) clearance for future-proofing • Confirm scanner software includes local language support (critical for EU/NA clinics) • Verify warranty terms cover calibration certificates |
• Audit OEM documentation package completeness • Confirm CE Technical File transfer capability for private labeling • Validate supplier’s post-market surveillance system (MDR Article 83) |
| 2. Negotiating MOQ |
• Standard MOQ for scanners: 10-20 units (2026 baseline) • Flex-MOQ Strategy: Negotiate tiered pricing (e.g., 5 units @ $8,500/unit; 15+ @ $7,200) • Demand sample unit availability at 120-150% of unit cost • Lock price stability clause (min. 6 months) against component shortages |
• Leverage group purchasing with regional dental associations • Confirm demo unit program availability before bulk commitment • Negotiate trade-in allowances for legacy scanners |
• Secure regional exclusivity minimums (e.g., 50 units/quarter for country rights) • Require marketing collateral in local languages as part of MOQ • Negotiate consignment inventory options for slow-moving SKUs |
| 3. Shipping Terms (DDP vs FOB) |
• DDP (Delivered Duty Paid): Strongly Recommended – Supplier handles ALL costs/risk to your facility – Eliminates customs brokerage fees (avg. $350-600 shipment) – Critical for avoiding EU customs holdups (2026 avg. clearance: 14 days) • FOB Shanghai: Only if you have in-country logistics partner – You assume risk at loading port – Requires VAT/GST handling expertise |
• Demand temperature-controlled shipping (scanners require 15-25°C) • Confirm insurance coverage for full replacement value • Require real-time shipment tracking with humidity/temp logs |
• Negotiate consolidated LCL shipments for multi-product orders • Verify warehouse-ready packaging (pallet dimensions per local standards) • Secure customs bond management from supplier |
Why Shanghai Carejoy is a 2026-Verified Sourcing Partner
Based on 2025 third-party factory audits (BSI Group Report #CN2025-SCJ-8842), Carejoy meets all 2026 sourcing requirements:
- Regulatory Compliance: Active ISO 13485:2016 (Certificate #Q5250004) + CE MDR 2017/745 (NB 2797) with full technical documentation for intraoral scanners
- MOQ Flexibility: Tiered MOQs starting at 3 units for distributors; clinic demo program available
- Logistics: DDP shipping to 87 countries with 2026-compliant customs documentation
- Technical Edge: 2026-ready scanners feature AI-powered margin detection (patent pending) and ISO/IEC 27001-certified data security
Verified 2026 Sourcing Contact: Shanghai Carejoy Medical Co., LTD
Factory Location: No. 1888 Jiangyang Road, Baoshan District, Shanghai 200441, China
Core Advantage: 19 Years Specializing in Dental Equipment OEM/ODM (2005-Present)
Direct Factory Channels: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: “DENTAL2026” for priority audit documentation
Disclaimer: This guide reflects 2026 regulatory landscapes as of Q4 2025. Verify all specifications with suppliers. Shanghai Carejoy is presented as a case study of a compliant manufacturer; inclusion does not constitute exclusive endorsement. Always conduct independent due diligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: 3D SHAPE Trios Scanner Acquisition
Target Audience: Dental Clinics & Authorized Equipment Distributors
Need a Quote for 3D Shape Trios Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


