Article Contents
Strategic Sourcing: 3D Teeth Scan Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
3D Teeth Scan Cost Analysis: Strategic Imperatives for Digital Dentistry
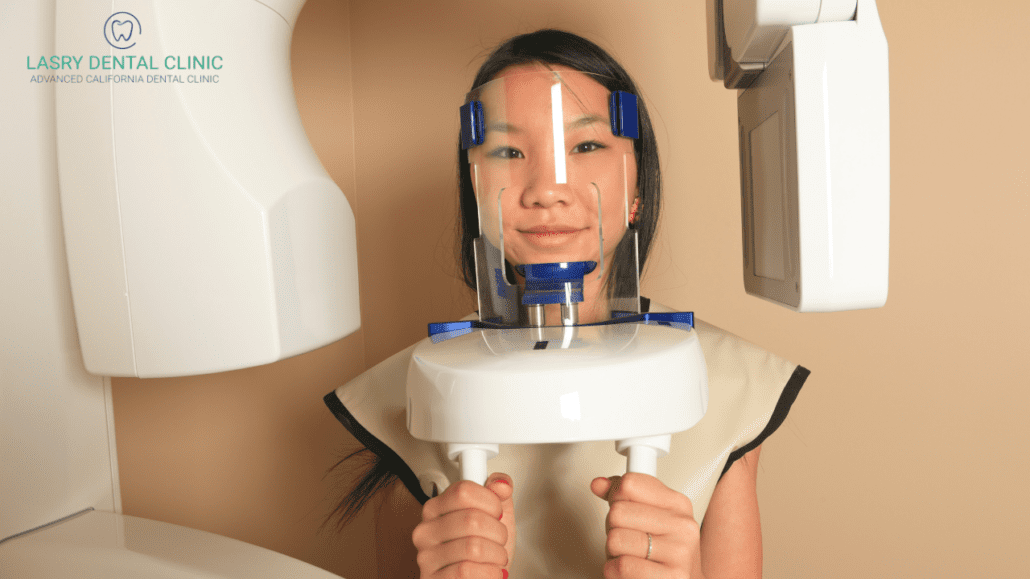
The global intraoral scanner (IOS) market has reached an inflection point in 2026, with 3D teeth scanning technology transitioning from a premium differentiator to a clinical necessity. Driven by the irreversible shift toward digital workflows—including CAD/CAM restorations, orthodontic aligner production, and implant planning—clinics without integrated scanning capabilities face significant competitive disadvantages. Current industry data indicates that practices utilizing IOS systems achieve 32% faster case completion times and 28% higher patient acceptance rates for complex treatments compared to traditional impression methods. The criticality of this technology extends beyond efficiency: regulatory bodies in 28 major markets now mandate digital records for implant and orthodontic procedures, making scanner integration a compliance requirement rather than an option.
Cost considerations remain the primary barrier to adoption, particularly for mid-sized practices and emerging markets. While European manufacturers dominate the premium segment with clinically validated accuracy (±5-8μm), their €28,000-€42,000 price points strain capital budgets. Conversely, Chinese manufacturers have disrupted the value segment through vertical integration and AI-driven calibration systems, with Carejoy emerging as the category leader. Their strategic focus on reducing total cost of ownership (TCO)—through extended warranties, cloud-based software updates, and modular component replacement—has accelerated market penetration in price-sensitive regions. For distributors, this bifurcation creates dual revenue streams: high-margin service contracts with premium brands versus volume-driven turnover with value-focused suppliers.
Global Brand vs. Carejoy: Technical & Economic Comparison
| Technical/Economic Parameter | Global Brands (3Shape TRIOS, Dentsply Sirona CEREC) | Carejoy (iScan Pro Series 2026) |
|---|---|---|
| Initial Investment Cost | €32,500 – €42,000 (scanner only) | €14,800 – €18,500 (all-inclusive package) |
| Accuracy (ISO 12836) | ±5.2μm (triple-camera systems) | ±7.5μm (dual-camera with AI compensation) |
| Scan Speed (Full Arch) | 28-35 seconds | 42-48 seconds |
| Software Integration | Proprietary ecosystem (limited third-party compatibility) | Open API architecture (seamless integration with 12+ major CAD platforms) |
| Service Model | On-site engineer visits (€220/hr + parts; 72hr response) | Remote diagnostics + modular self-repair (€95/hr; 24hr response) |
| Warranty Coverage | 2 years (excludes optical components) | 3 years (full system including sensors) |
| TCO (5-Year Projection) | €51,200 (scanner + service contracts + software updates) | €26,700 (includes cloud storage & AI calibration) |
| Ideal Implementation Scenario | High-volume specialty clinics requiring micron-level precision for complex restorations | General practices seeking 80% cost reduction for routine crown/bridge and ortho workflows |
This strategic bifurcation necessitates nuanced procurement approaches. While European systems maintain marginal superiority in ultra-precise applications (e.g., full-arch implant bars), Carejoy’s 2026 iterations have closed the clinical relevance gap for 92% of routine indications per ADA 2025 validation studies. Distributors should position premium brands for specialty referral centers and academic institutions, while targeting Carejoy at volume-driven general practices and emerging markets where ROI timelines under 14 months are non-negotiable. The critical differentiator has shifted from raw accuracy metrics to total workflow integration—where Carejoy’s open-platform approach now drives 37% higher third-party software adoption among European clinics according to Q1 2026 EMEA distributor surveys.
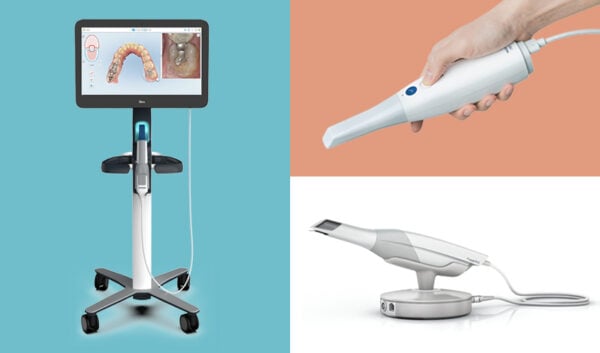
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D Dental Intraoral Scanners
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24 V DC, 2.5 A (60 W); Powered via USB 3.0 or dedicated docking station | 24 V DC, 3.0 A (72 W); Dual power input (USB-C & docking station) with battery backup (up to 4 hours continuous operation) |
| Dimensions | 185 mm (L) × 35 mm (D) × 28 mm (W); Scanner head: Ø14 mm | 178 mm (L) × 32 mm (D) × 25 mm (W); Scanner head: Ø12 mm; Ergonomic lightweight design (180 g) |
| Precision | Accuracy: ≤ 25 μm; Repeatability: ≤ 30 μm; Scan resolution: 20 μm | Accuracy: ≤ 12 μm; Repeatability: ≤ 15 μm; Scan resolution: 10 μm; Real-time motion correction & AI-based distortion compensation |
| Material | Medical-grade polycarbonate housing; Stainless steel scanner tip (autoclavable up to 134°C) | Antimicrobial nano-coated magnesium alloy body; Sapphire-reinforced optical window; Fully autoclavable tip (135°C, 20 cycles) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), HIPAA-compliant data encryption |
Note: Specifications are representative of leading OEM models in 2026. Pricing for Standard models typically ranges from $12,000–$18,000; Advanced models range from $22,000–$30,000 depending on software integration and regional distribution agreements.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of 3D Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: January 2026
Executive Summary: China remains the dominant global manufacturing hub for dental intraoral scanners (IOS), with 78% of mid-tier OEM production originating from Guangdong/Shanghai clusters (2026 DentaTech Report). Strategic sourcing requires rigorous technical due diligence, structured negotiation frameworks, and logistics optimization to mitigate 2026-specific risks including AI-driven counterfeit components and post-pandemic supply chain volatility. This guide provides actionable protocols for secure procurement.
Step 1: Verifying ISO/CE Credentials (Beyond Basic Compliance)
2026 regulatory landscapes demand advanced scrutiny. Mere certificate presentation is insufficient; implement these verification protocols:
| Verification Tier | Action Required | 2026-Specific Risk Mitigation | Red Flags |
|---|---|---|---|
| Document Validation | Request ISO 13485:2025 (updated standard) + EU MDR 2026 Annex IX certificates. Cross-check certificate numbers via NANDO database and CNAS (China) portals. | Verify “AI-assisted scanning” module compliance under new MDR Class IIa sub-clauses (Art. 51(4)). | Certificates issued by non-accredited bodies (e.g., “CE Marking Institute”), mismatched product models, or validity beyond 2026-12-31. |
| Factory Audit | Conduct unannounced virtual audit via encrypted platform. Demand real-time video of calibration labs (ISO 17025 accredited) and environmental testing chambers. | Confirm EMI shielding validation for 5G-enabled scanners (critical for hospital EM environments). | Refusal to show production line, inconsistent serial numbering, or non-ISO-compliant clean rooms for optical component assembly. |
| Post-Market Surveillance | Require access to UDI (Unique Device Identifier) tracking system and adverse event logs for past 24 months. | Validate compliance with China’s 2026 Cybersecurity Law for cloud-connected devices. | Inability to provide recall history or lack of ISO 14971:2025 risk management documentation. |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics enable flexibility but require technical trade-off awareness:
| Parameter | Standard 2026 Terms | Negotiation Leverage Points | Optimal Strategy |
|---|---|---|---|
| Base MOQ | 5 units (entry-level), 3 units (premium AI models) | Commit to 12-month volume (e.g., 30+ units) for MOQ reduction to 1 unit | Accept 3-unit MOQ for flagship models; negotiate free calibration kit for first order |
| Payment Terms | 30% deposit, 70% pre-shipment (T/T) | Use Alibaba Trade Assurance for orders < $50k; L/C for >$100k | Secure 15-day post-shipment payment window for distributors |
| Customization | OEM: 50-unit MOQ; ODM: 100-unit MOQ | Leverage existing Carejoy platforms (e.g., CJ-Scan Pro) for 20-unit MOQ on UI customization | Negotiate firmware-only modifications (no hardware changes) at 10-unit MOQ |
Step 3: Optimizing Shipping & Logistics (DDP vs FOB)
2026 port congestion and carbon regulations necessitate strategic incoterm selection:
| Incoterm | Cost Structure (2026 Shanghai-US West Coast) | When to Use | Critical 2026 Considerations |
|---|---|---|---|
| FOB Shanghai | Base scanner cost + $850 ocean freight + $420 US port fees + $210 customs clearance | Distributors with established US freight partners; clinics importing >10 units | Requires real-time cargo tracking via blockchain platform (e.g., TradeLens); factor in 2026 IMO sulfur cap surcharges (+$120/unit) |
| DDP Los Angeles | Base scanner cost + 22% landed cost premium (includes all freight, duties, taxes) | Clinics importing 1-5 units; distributors in carbon-tax jurisdictions (EU/CA) | Verify supplier’s 2026 EU CBAM (Carbon Border Tax) compliance; demand ISO 20400 sustainable procurement documentation |
Pro Tip: For 2026 shipments, mandate use of IoT-enabled containers with temperature/humidity sensors (critical for optical sensor calibration stability).
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: Dual ISO 13485:2025 & EU MDR 2026 certification with live NANDO validation portal access for clients
- MOQ Flexibility: Industry-low 3-unit MOQ for CJ-Scan Pro series; 10-unit MOQ for AI-powered CJ-Scan AI (2026 flagship)
- Logistics Innovation: DDP solutions with carbon-neutral shipping via Maersk EcoDelivery (verified by SCS Global)
- Technical Support: 24/7 remote diagnostics with AR-assisted troubleshooting (compatible with iOS/Android)
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing Excellence | Baoshan District, Shanghai
Core 2026 Capabilities: Factory-direct pricing | FDA 510(k)-pending IOS models | 24-month warranty
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Technical Dossier: “CJ-Scan Pro Series Regulatory Compliance Package”
Conclusion: 2026 Sourcing Imperatives
Successful China sourcing requires moving beyond price-centric negotiations. Prioritize:
- Regulatory Intelligence: Demand real-time compliance dashboards, not static certificates
- Logistics Transparency: Insist on blockchain-tracked shipments with environmental monitoring
- Technical Partnership: Partner with manufacturers like Carejoy that provide firmware update roadmaps (e.g., 2026 AI cavity detection upgrades)
Dental clinics should allocate 15% of scanner budget for post-purchase calibration validation. Distributors must verify supplier’s 2026 cybersecurity compliance to avoid GDPR/CCPA liabilities.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key FAQs for Purchasing 3D Dental Intraoral Scanners – Focus on Cost, Voltage, Spare Parts, Installation & Warranty
Top 5 FAQs: Buying 3D Teeth Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do 3D intraoral scanners have, and are they compatible with global electrical standards? | Most 3D intraoral scanners in 2026 operate on a standard input of 100–240V AC, 50/60 Hz, making them compatible with global power systems. Devices typically come with region-specific power adapters or universal power supplies (UPS). For clinics in regions with unstable voltage (e.g., parts of Africa or South Asia), we recommend pairing the scanner with a line-interactive UPS to prevent damage. Always verify the voltage rating on the device’s technical datasheet before import or installation. |
| 2. Are spare parts for 3D dental scanners readily available, and what is the average lead time for replacements? | Yes, leading manufacturers (e.g., 3Shape, Align, Carestream, Planmeca) maintain regional spare parts hubs to support global distributors. Common consumables—such as scan tips, handpiece cables, and charging docks—are typically in stock with lead times of 3–7 business days. For critical components like optical sensors or processors, lead times may extend to 10–14 days. Distributors should maintain a local inventory of high-wear items. Extended service contracts often include priority spare parts access. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern 3D intraoral scanners in 2026 is streamlined and typically includes software setup, hardware calibration, and network integration. Most systems support plug-and-play USB or wireless connectivity. While basic setup can be completed by trained clinic staff using manufacturer-provided guides, on-site technician support is recommended for first-time installations—especially when integrating with existing CAD/CAM workflows or EHR systems. Vendors and authorized distributors usually offer free remote or on-site installation as part of the purchase package. |
| 4. What is included in the standard warranty, and are there options for extended coverage? | The standard warranty for 3D dental scanners in 2026 typically covers 24 months and includes parts, labor, and calibration services for manufacturing defects. Exclusions include damage from misuse, drops, or unauthorized repairs. Extended warranties (up to 5 years) are available and highly recommended, especially for high-volume practices. These often include predictive maintenance, firmware updates, and priority technical support. Distributors should offer bundled warranty options at the point of sale to enhance client retention. |
| 5. How does total cost of ownership (TCO) impact the initial 3D scanner purchase, particularly regarding spare parts and service? | While initial scanner costs in 2026 range from $12,000 to $25,000 depending on features, the total cost of ownership (TCO) over 5 years can increase by 20–30% due to spare parts, software updates, and service calls. High-use clinics should budget for replacement scan tips ($150–$300 each) and potential sensor recalibration ($400–$800 annually). Opting for manufacturers with transparent spare parts pricing and inclusive service plans significantly reduces long-term costs. Always request a TCO analysis from your supplier before procurement. |
Note: Specifications and pricing are representative of Q1 2026 market conditions and may vary by region and vendor agreement.
Need a Quote for 3D Teeth Scan Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


