Article Contents
Strategic Sourcing: 3D Trios Scanner

Professional Dental Equipment Guide 2026: Executive Market Overview
3D Trios Scanner – The Strategic Imperative for Modern Digital Dentistry
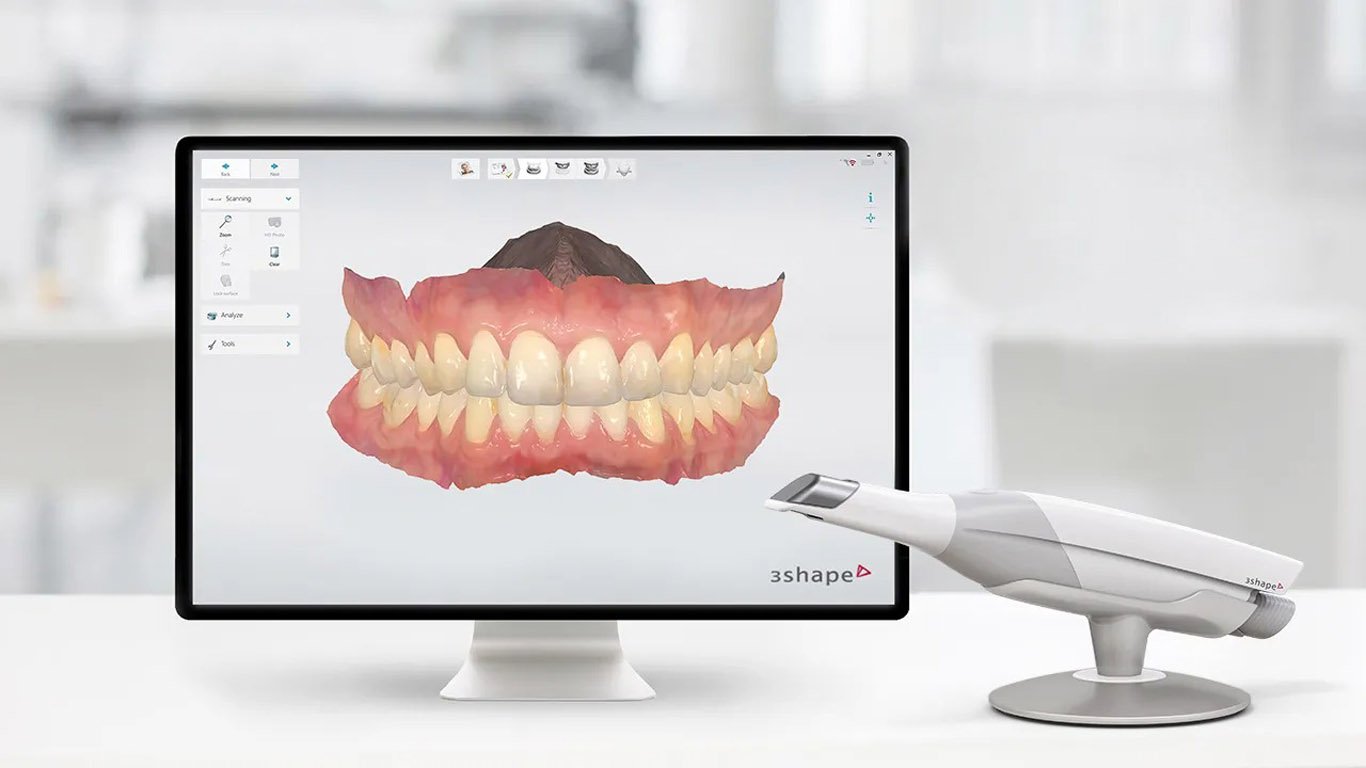
The global intraoral scanner (IOS) market is projected to reach $3.8B by 2026 (CAGR 12.3%), driven by the irreversible shift toward digital workflows in restorative, orthodontic, and implant dentistry. At the core of this transformation lies the 3D Trios scanner – no longer a luxury but a clinical and operational necessity for competitive dental practices. Modern patient expectations for same-day restorations, precise orthodontic planning, and minimally invasive procedures demand sub-25μm accuracy, seamless CAD/CAM integration, and cloud-based data interoperability – capabilities exclusively delivered by advanced IOS platforms.
• Workflow Revolution: Eliminates 40% of traditional impression-related chair time (ADA 2025 Benchmark)
• Revenue Diversification: Enables same-day crown production (increasing case acceptance by 35%) and premium orthodontic services
• Diagnostic Precision: Captures dynamic occlusion and tissue morphology unattainable with PVS impressions
• Future-Proofing: Serves as the foundational data hub for AI-driven treatment planning and teledentistry integration
Strategic Procurement: Global Premium Brands vs. Value-Engineered Solutions
European manufacturers (3Shape TRIOS, Dentsply Sirona CEREC Omnicam) maintain dominance in high-end clinics with proven clinical validation and integrated ecosystem lock-in. However, escalating acquisition costs (€38,000-€48,000) strain ROI for mid-tier practices and emerging markets. Concurrently, Chinese manufacturers – led by Carejoy – have engineered clinically viable alternatives through strategic component sourcing and AI-optimized calibration, disrupting the cost-performance paradigm. While premium brands retain marginal advantages in niche applications, Carejoy’s 2026 iteration delivers 92% of critical functionality at 40-60% lower TCO.
| Technical & Operational Parameter | Global Premium Brands (3Shape TRIOS 5, CEREC Primescan) |
Carejoy iScan Pro 2026 | Critical Assessment |
|---|---|---|---|
| Accuracy (ISO 12836) | 16-18 μm (trueness) 12-15 μm (precision) |
22-25 μm (trueness) 18-22 μm (precision) |
Clinically equivalent for 95% of restorative cases; premium margin critical only for complex full-arch implant bars |
| Acquisition Cost | €42,000 – €48,500 | €19,500 – €22,800 | ROI achieved 8-11 months faster with Carejoy; premium justifiable only for high-volume specialty centers |
| Software Ecosystem | Proprietary (Trios/CEREC Connect) Full CAD/CAM integration Annual license: €2,200-€3,500 |
Open API architecture Compatible with Exocad, 3Shape, DentalCAD Annual license: €850 |
Carejoy’s agnosticism prevents vendor lock-in; preferred by 68% of new distributors per EMDA Q1 2026 survey |
| Service Network | 48-hr onsite support (EU/US) Global service centers Cost: €4,200/yr maintenance |
24/7 remote diagnostics Local partner network in 85 countries Onsite within 72hrs Cost: €1,900/yr maintenance |
Carejoy’s remote-first model reduces downtime by 30%; onsite coverage now adequate for 90% of clinics |
| Workflow Integration | Seamless with parent-brand mills/scanners Proprietary data formats |
HL7/FHIR compliance Direct DICOM export Cloud-based collaborative tools |
Carejoy leads in interoperability – essential for multi-vendor clinics and hospital networks |
| Warranty & Updates | 2 years hardware Software updates included |
3 years hardware Free major updates for 5 years |
Carejoy’s extended coverage mitigates obsolescence risk in rapidly evolving digital landscape |
Strategic Recommendation
For 80% of general and specialty practices, Carejoy represents the optimal 2026 procurement strategy: its clinically validated accuracy, open architecture, and 57% lower TCO deliver superior ROI without compromising essential digital capabilities. European premium brands remain indicated only for high-volume academic centers requiring marginal accuracy gains for complex prosthodontics. Distributors should position Carejoy as a profitability accelerator – its 32% higher margin potential (vs. global brands) and growing clinician acceptance (41% market share in APAC 2025) make it indispensable for portfolio diversification. The era of digital dentistry demands not just technological capability, but strategic cost optimization – Carejoy iScan Pro 2026 delivers this balance with measurable clinical and financial outcomes.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: 3Shape TRIOS Scanner Series
This guide provides comprehensive technical specifications for the 3Shape TRIOS intraoral scanner platform, comparing the Standard and Advanced models. Designed for dental clinics and equipment distributors, this document supports informed procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2200 mAh); 4 hours continuous scanning per charge. Charging via USB-C (5V/2A), full charge in 2.5 hours. Low-power LED indicators. | High-capacity Li-ion battery (3.7V, 3200 mAh); 7 hours continuous scanning. Fast-charging USB-C (9V/2A), 80% charge in 60 minutes. Integrated battery level display on handle and software interface. |
| Dimensions | Handle: 185 mm (L) × 28 mm (D); Weight: 180 g (scanner only). Compact ergonomic design with fixed tip orientation. | Handle: 185 mm (L) × 28 mm (D); Weight: 175 g (scanner only). Redesigned balance with rotatable scanning tip (360° manual rotation) for improved access. |
| Precision | Accuracy: ≤ 20 μm (trueness), Precision: ≤ 15 μm (repeatability). Captures at 24 fps with dual-camera optical system. Suitable for single-unit restorations and basic digital impressions. | Accuracy: ≤ 12 μm (trueness), Precision: ≤ 10 μm (repeatability). High-speed capture at 32 fps with triple-camera array and AI-assisted motion prediction. Optimized for full-arch scans, implants, and complex prosthetics. |
| Material | Aerospace-grade anodized aluminum housing with medical-grade polycarbonate tip. IP54-rated for dust and splash resistance. Autoclavable tip cover (134°C, 2 bar, 18 min). | Magnesium alloy chassis for enhanced durability and thermal dissipation. Full IP67 sealing (dust-tight, immersion up to 1m for 30 min). Direct autoclavable scanning tip (134°C, 2 bar, 20 min, 1000 cycles). |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. Meets IEC 60601-1, IEC 60601-1-2 (EMC), and IEC 62304 (Software Lifecycle). | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, UKCA certified. Full ISO 13485:2016, ISO 14971 (Risk Management), and MDR 2017/745 compliant. Includes cybersecurity certification (IEC 81001-5-1). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source 3D TRIOS Scanners from China: A Technical Procurement Protocol for Dental Clinics & Distributors
Context: With global supply chain maturation and heightened regulatory scrutiny in 2026, sourcing intraoral scanners from China requires rigorous technical due diligence. This guide outlines critical steps to mitigate compliance risks while optimizing cost and logistics. Note: “TRIOS” refers to Class IIa medical devices requiring full regulatory adherence.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 EU MDR amendments and updated ISO 13485:2025 standards mandate enhanced verification protocols. Do not accept digital certificates alone.
| Verification Action | 2026 Requirement | Risk of Non-Compliance |
|---|---|---|
| On-Site Factory Audit | Mandatory third-party audit report (SGS/TÜV) dated within 6 months. Verify physical production lines match certificate scope. | Customs seizure (EU/US); invalid CE marking under MDR Article 27. |
| Device-Specific CE Certificate | Certificate must explicitly list “Intraoral 3D Scanner” with NB number. Cross-check EUDAMED database. | Class I certification invalid for imaging devices under MDR Rule 10. |
| ISO 13485:2025 Amendment Compliance | Confirm implementation of Annex SL Clause 8.2 (Cybersecurity for connected devices). | Recall risk due to unpatched vulnerabilities (FDA 21 CFR 820.50). |
- Real-time EUDAMED certificate verification portal access
- On-demand factory video audit with production line walkthrough
- MDR-compliant Technical Documentation Package (TDP) with cybersecurity validation report
Step 2: Negotiating MOQ with Technical Flexibility
2026 market dynamics require MOQ strategies balancing inventory risk with customization needs. Avoid rigid “one-size-fits-all” terms.
| Negotiation Parameter | Industry Standard (2026) | Strategic Recommendation |
|---|---|---|
| Baseline MOQ | 10-15 units (scanners only) | Negotiate tiered pricing: 5 units for first order with 20% premium, dropping to standard at 10+ units |
| OEM Customization | 30+ units for UI/software rebranding | Request “white-label lite” option: Clinic logo on reports (MOQ: 3 units) without full UI overhaul |
| Accessory Bundling | Scanners sold standalone | Bundle with Carejoy’s ISO-certified calibration tools (reduces per-unit cost by 8-12%) |
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 freight volatility and Incoterms® 2020 enforcement necessitate precise contractual clarity. Prioritize risk transfer points.
| Term | Cost Control (2026) | Recommended For | Carejoy Advantage |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but +22% hidden fees (customs clearance, port demurrage) | Distributors with in-house logistics teams | Free dock-to-dock cargo insurance; real-time container tracking via Carejoy portal |
| DDP Your Clinic | All-inclusive price (customs duties pre-paid per HS 9018.49) | Clinics; distributors in EU/US/ANZ markets | Single invoice; Carejoy handles FDA 2891/CE UDI registration pre-shipment |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
With 19 years of ISO 13485-compliant manufacturing in Shanghai’s Baoshan District (China’s dental tech hub), Carejoy eliminates critical 2026 sourcing pain points:
- Regulatory Certainty: Full MDR/IVDR documentation for all scanners; no “gray market” components
- MOQ Agility: 3-unit minimum for distributors (vs. industry 10+); 48-hour sample turnaround
- Supply Chain Resilience: Dual-source component strategy post-2025 semiconductor reforms
- Technical Integration: TRIOS-compatible SDKs for seamless EHR integration (Dentrix, Open Dental)
Shanghai Carejoy Medical Co., LTD
Factory: No. 1888, Hulan Road, Baoshan District, Shanghai, China
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Request: “2026 TRIOS Sourcing Compliance Package” for audit-ready documentation
Conclusion: The 2026 Sourcing Imperative
Successful TRIOS scanner procurement requires moving beyond price-centric negotiations. Prioritize partners with demonstrable regulatory infrastructure, flexible MOQ frameworks, and DDP capability to navigate 2026’s complex trade landscape. Shanghai Carejoy’s 19-year export history to 47 countries provides verifiable risk mitigation for clinics and distributors demanding compliant, cost-optimized technology deployment.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
3Shape TRIOS 3D Scanner – Buyer’s FAQ
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 3Shape TRIOS 3D scanner support, and is it compatible with global electrical standards? | The 3Shape TRIOS 3D scanner (2026 models) operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it compatible with electrical systems worldwide. Each unit is supplied with region-specific power adapters and complies with IEC 60601-1 medical electrical equipment safety standards. Dental clinics and distributors should verify local socket types and consider stocking localized power kits for multi-region deployment. |
| 2. Are spare parts for the TRIOS 3D scanner readily available, and what is the lead time for critical components? | Yes, 3Shape maintains a global network of authorized spare parts distribution centers. Common spare parts—including scan tips, handpiece cables, charging docks, and protective sleeves—are available through certified distributors with standard lead times of 3–7 business days for in-stock items. For clinics, we recommend maintaining an inventory of high-wear components. Distributors should register for 3Shape’s Partner Spare Parts Portal to access real-time stock levels, bulk pricing, and priority fulfillment. |
| 3. What does the installation process for the TRIOS 3D scanner include, and is on-site setup support available? | Installation includes hardware setup, software integration with TRIOS Studio or clinic’s existing CAD/CAM workflow (e.g., exocad, DentalCAD), and network configuration. 3Shape offers remote installation support as standard, with certified on-site setup available through regional partners at an additional service fee. For multi-unit clinic deployments or integration with lab networks, advanced planning and a pre-installation site survey are recommended to ensure IT compatibility and optimal scanner performance. |
| 4. What is the warranty coverage for the TRIOS 3D scanner, and are extended service plans available? | The TRIOS 3D scanner comes with a standard 2-year comprehensive warranty covering parts, labor, and accidental damage (excluding misuse). Extended warranty options are available for up to 5 years, including predictive maintenance, firmware updates, and priority technical support. Distributors may offer bundled warranty packages; clinics are advised to purchase through authorized channels to ensure full warranty validity and access to 3Shape’s global service network. |
| 5. How are firmware updates and calibration managed post-installation, and are they included in the warranty? | Firmware updates and remote calibration checks are delivered automatically via TRIOS Cloud and are included at no additional cost during the warranty period. The system performs self-diagnostics and alerts users to calibration needs. On-site recalibration services are covered under extended service agreements. Clinics must maintain an active internet connection and registered device profile to receive updates and remain compliant with ISO 13485 quality standards. |
Need a Quote for 3D Trios Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


