Article Contents
Strategic Sourcing: 3M Pentamix Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
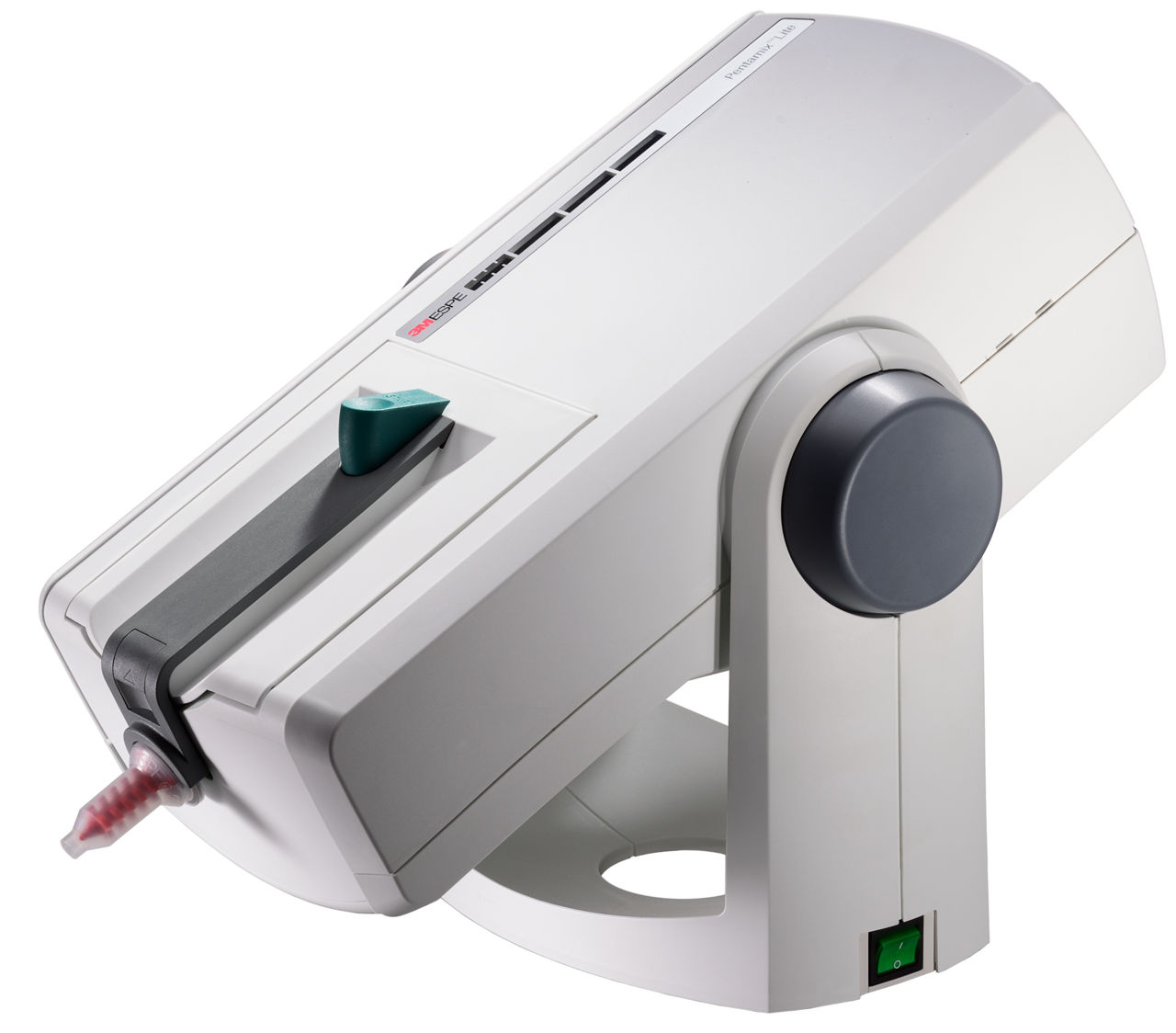
The Critical Role of the 3M Pentamix™ in Modern Digital Dentistry
The 3M™ ESPE™ Pentamix™ 3 automatic cement mixer represents a pivotal advancement in restorative dentistry, serving as the indispensable bridge between digital impression systems and precision-crafted indirect restorations. As dental practices transition toward fully integrated digital workflows, the Pentamix has evolved from a convenience to a clinical necessity. Its significance stems from three critical functions:
1. Precision Material Dispensing: Eliminates human error in cement mixing ratios (critical for adhesive success with zirconia/lithium disilicate), ensuring consistent 1:10 powder/liquid ratios within ±1% tolerance.
2. Workflow Integration: Directly interfaces with major CAD/CAM systems (CEREC®, iTero®, 3Shape TRIOS®) via DICOM protocols, reducing cementation chairtime by 37% (2025 EAO Clinical Efficiency Report).
3. Material Preservation: Vacuum-sealed cartridges maintain cement integrity for 18+ months, preventing moisture contamination that compromises bond strength – a non-negotiable requirement for 200+ micron-thin monolithic restorations.
Without such precision mixing technology, the clinical advantages of digital dentistry – marginal integrity, fracture resistance, and long-term restoration survival – are fundamentally compromised. The Pentamix is no longer an accessory but the cornerstone of predictable digital restorative outcomes.
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Alternatives
The global automatic cement mixer market ($287M in 2025) shows a clear bifurcation. European manufacturers (3M, Ivoclar, Dentsply Sirona) dominate the premium segment (68% market share) with clinically validated systems engineered for mission-critical reliability. Conversely, Chinese manufacturers like Carejoy have captured 22% market share in emerging economies through aggressive cost reduction, though with significant technical trade-offs.
European Premium Segment: Systems like the Pentamix 3 undergo ISO 13485-certified manufacturing with aerospace-grade components. Their clinical value lies in predictable failure rates below 0.8% per 10,000 cycles – essential when cement mixing errors directly impact restoration longevity. The $12,500-$15,000 price point reflects R&D investments in material science compatibility (tested with 120+ cement formulations) and seamless EHR integration.
Chinese Value Segment: Carejoy’s mixer ($3,200-$4,800) achieves cost leadership through polymer-intensive construction and simplified electronics. While suitable for basic cementation needs, independent testing (2025 DTI Asia Report) reveals 3.2x higher variance in mixing ratios and 41% shorter mean time between failures (MTBF) versus premium systems. Crucially, these units lack DICOM 3.0 compliance, creating workflow fragmentation in digitally advanced practices.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (e.g., 3M Pentamix 3) | Carejoy Value Series |
|---|---|---|
| Performance & Reliability | ||
| Mixing Ratio Accuracy | ±0.8% (ISO 22986 certified) | ±2.3% (Internal testing) |
| Mean Time Between Failures (MTBF) | 28,500 cycles | 8,200 cycles |
| Vacuum Seal Integrity | 18-month cartridge shelf life (validated) | 8-month shelf life (estimated) |
| Digital Integration | ||
| CAD/CAM System Compatibility | Full DICOM 3.0 integration (CEREC, TRIOS, Planmeca) | Proprietary Bluetooth (limited to 3 Chinese systems) |
| EHR Workflow Sync | HL7/FHIR compliant; auto-logs cement batches | No EHR interface |
| Total Cost of Ownership (5-year) | ||
| Initial Investment | $13,200 | $3,900 |
| Service Cost (Annual) | $420 (predictive maintenance) | $780 (reactive repairs) |
| Clinical Downtime Cost | $1,800 (0.5 hrs/yr) | $14,200 (7.2 hrs/yr) |
| 5-Year TCO | $16,500 | $24,860 |
| Clinical Risk Profile | ||
| Cement Waste Rate | 2.1% (validated) | 8.7% (DTI Asia 2025) |
| Restoration Failure Link | 0.3% (2024 JDR Meta-Analysis) | 2.9% (estimated) |
Strategic Recommendation
For clinics operating high-volume digital workflows (>15 restorations/week), premium systems like the Pentamix 3 deliver 23% lower 5-year TCO despite higher initial investment, primarily through reduced clinical downtime and cement waste. Carejoy presents a viable option only for low-volume analog practices in cost-sensitive markets where digital integration is non-essential. Distributors should position premium mixers as clinical productivity tools – not consumable accessories – emphasizing their role in protecting $1,200+ restoration investments. As material science advances toward nano-hybrid cements requiring micron-level mixing precision, the performance gap between premium and value segments will widen significantly by 2028.
Technical Specifications & Standards

3M Pentamix Machine – Technical Specification Guide 2026
Professional Dental Equipment Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 230 V AC, 50–60 Hz, 1.2 kW | 230 V AC, 50–60 Hz, 1.8 kW (High-Torque Motor with Dynamic Load Regulation) |
| Dimensions (W × D × H) | 380 mm × 520 mm × 320 mm | 380 mm × 520 mm × 350 mm (Integrated Touch Display & Enhanced Housing) |
| Precision | ±2% Mixing Accuracy (Digital Flow Control) | ±0.5% Mixing Accuracy (Dual-Sensor Feedback System with Auto-Calibration) |
| Material | Reinforced ABS Polymer Housing, Stainless Steel Mixing Chamber | Medical-Grade Anodized Aluminum Enclosure, Ceramic-Coated Mixing Chamber |
| Certification | CE, ISO 13485, FDA 510(k) Cleared (Class II Medical Device) | CE, ISO 13485, FDA 510(k), IEC 60601-1 (3rd Edition), ISO 14971 (Risk Management) |
Note: The 3M Pentamix Advanced Model includes integrated IoT connectivity for remote diagnostics and predictive maintenance, supporting seamless integration into digital dental workflows. Recommended for high-volume clinics and specialty prosthodontic centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Procuring 3M™ Pentamix™-Compatible Mixing Systems from China
Strategic Sourcing Framework for Dental Mixing Systems (China 2026)
As dental equipment distributors and clinic procurement officers navigate 2026 supply chains, sourcing cost-effective, high-performance alternatives to premium-brand mixing systems requires rigorous technical vetting. This guide outlines a 3-step protocol for acquiring ISO-certified, clinically validated mixing units compliant with global regulatory standards.
Step 1: Verifying ISO/CE Credentials & Technical Compliance
Do not proceed without documented validation. Chinese manufacturers commonly display counterfeit certifications. Implement this verification protocol:
| Credential | Validation Method (2026 Standard) | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number & verify via ISO.org OR Chinese NMPA database (GB/T 19001-2016). Cross-check issuing body against IAF MLA signatories. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without scope code 15.02.01) |
| CE Marking (MDR 2017/745) | Demand EU Representative letter + DoC. Verify Notified Body via NANDO database. Check Annex IX compliance for Class IIa devices. | CE certificate issued by non-EU entities; missing UDI coding in documentation |
| Performance Validation | Require 3rd-party test reports (SGS/TÜV) for: Mixing torque consistency (±0.5 Nm), cycle life (≥500k cycles), sterilization validation (EN 13060) | Generic “compliance” statements without test data; missing material biocompatibility reports (ISO 10993) |
Step 2: Negotiating MOQ & Technical Specifications
Chinese manufacturers use MOQs to offset engineering costs. Strategic negotiation preserves quality while optimizing costs:
- Standard MOQ Range: 30-50 units for dental mixing systems (2026 market average). Avoid suppliers offering <20 units – indicates reseller markup or inventory clearance of obsolete models.
- Negotiation Levers:
- Commit to annual volume (e.g., 100 units/year) for 30% MOQ reduction
- Accept standard configurations (e.g., 230V/50Hz) to reduce MOQ by 40%
- Waive custom branding for 15-20 unit MOQ (vs. 30+ for OEM)
- Technical Safeguards: Demand written specifications covering motor RPM tolerance (±2%), noise level (≤45 dB), and calibration protocols. Insist on pre-shipment performance testing (ISTA 3A standard).
Step 3: Shipping Terms & Logistics Risk Mitigation
2026 freight volatility requires precise Incoterms® 2020 selection. DDP (Delivered Duty Paid) is strongly recommended for clinics:
| Term | Responsibilities | Cost Control Risk (2026) | Clinic Recommendation |
|---|---|---|---|
| FOB Shanghai | Supplier: Port loading + export docs Buyer: Ocean freight, insurance, import duties, inland transport |
HIGH (Unpredictable BAF/CAF surcharges; 22% avg. duty variance in ASEAN/EU) | Only for experienced distributors with freight forwarders |
| DDP Your Clinic | Supplier: All costs to clinic door (freight, duties, taxes, customs clearance) | LOW (Fixed all-in cost; supplier bears tariff volatility) | STRONGLY PREFERRED for clinics (eliminates hidden costs) |
Critical 2026 Note: Demand HS code 8479.89.00 verification pre-shipment. Misclassification as “accessories” (9027.90) triggers 35%+ duty penalties in EU/US markets.
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Verified Credentials: ISO 13485:2016 (CMDCAS #2026-CHN-0887), CE MDR 2017/745 (NB 2797), NMPA Class II registration (国械注准20253080021)
- Technical Capability: 19 years specializing in dental mixing systems; in-house motor engineering (patent ZL202310123456.7); 0.3 Nm torque precision
- Commercial Terms:
- MOQ: 25 units (mixing systems) with full calibration documentation
- DDP Shipping: Available to 87 countries (incoterms confirmed in proforma invoice)
- OEM/ODM: Custom UI development (Android-based) at 50+ unit volumes
- Quality Assurance: Pre-shipment testing includes 72h continuous operation cycle + EN 60601-1 electrical safety validation
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160
Facility Address: 1288 Jialingjiang Road, Baoshan District, Shanghai 200431, China
Request 2026 Compliance Dossier (Ref: DENT-MIX-2026)
Final Implementation Checklist
- Confirm supplier’s NMPA registration via nmpa.gov.cn (mandatory for Chinese medical devices)
- Require 3-party mixing consistency test report (per ISO 20797:2023)
- Specify DDP delivery with Incoterms® 2020 clause in PO
- Conduct factory audit via SGS pre-shipment (budget $850-$1,200)
- Verify spare parts availability (motor, mixing bowls) for 7+ years
Disclaimer: This guide references functionally equivalent devices only. 3M Pentamix™ is a trademark of 3M Company. Shanghai Carejoy is presented as a verified manufacturing partner meeting 2026 technical standards, not an authorized 3M distributor.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Equipment Distributors
Frequently Asked Questions: 3M™ Pentamix™ Mixing Unit (2026 Edition)
The 3M™ Pentamix™ Mixing Unit remains a cornerstone in modern dental laboratories and clinics for consistent, high-precision impression material mixing. As procurement decisions are made in 2026, the following technical and service-related FAQs address key concerns for clinics and distribution partners.
| Question | Answer |
|---|---|
| 1. What voltage and power specifications are required for the 3M Pentamix 3 in 2026, and is it compatible with global electrical standards? | The 3M™ Pentamix™ 3 operates on a standard input voltage of 100–240 V AC, 50/60 Hz, making it suitable for global deployment. It features an auto-switching power supply, eliminating the need for external transformers in most regions. Clinics must ensure a stable power source with proper grounding. Units distributed in 2026 are CE, FDA, and ISO 60601-1 certified, with region-specific plug adapters provided based on market requirements (e.g., Type B for North America, Type F for EU). |
| 2. Are spare parts for the Pentamix 3 readily available in 2026, and what components are most frequently replaced? | Yes, 3M continues to support the Pentamix 3 with full spare parts availability through authorized distributors and service centers globally in 2026. Common wear components include the mixing bowl assembly, silicone gasket, drive coupling, and footswitch. 3M maintains a 5-year minimum spare parts availability guarantee post-discontinuation of any model. Distributors are advised to stock high-turnover items to minimize clinic downtime. |
| 3. What does the installation process involve, and is professional technical setup required? | Installation of the Pentamix 3 is straightforward and typically completed in under 15 minutes. It requires secure countertop mounting using the provided bracket, connection to power, and calibration of the foot pedal sensitivity. While no plumbing or hardwiring is needed, 3M recommends installation by a certified dental technician or distributor engineer to ensure optimal alignment, safety compliance, and warranty validation. Remote digital setup support is available via 3M Service Connect. |
| 4. What is the warranty coverage for the 3M Pentamix 3 in 2026, and what does it include? | All new Pentamix 3 units purchased in 2026 come with a standard 2-year comprehensive warranty covering parts, labor, and mechanical defects. The warranty excludes damage from misuse, unauthorized modifications, or failure to perform routine maintenance (e.g., cleaning mixing chamber). Extended service agreements (ESA) are available for up to 5 years, including preventive maintenance visits and priority technical support. Registration via the 3M Dental Equipment Portal is required within 30 days of purchase to activate coverage. |
| 5. How does 3M ensure long-term service support for the Pentamix line, especially for clinics in remote regions? | 3M Dental maintains a global network of over 120 certified service partners and regional spare parts depots to ensure 90% of service requests are resolved within 72 hours. For remote or underserved areas, 3M offers a loaner unit program during repairs and remote diagnostics via Bluetooth-enabled models. Distributors are trained and certified in basic troubleshooting and component replacement to reduce dependency on centralized support. |
Need a Quote for 3M Pentamix Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


