Article Contents
Strategic Sourcing: 3Shape Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
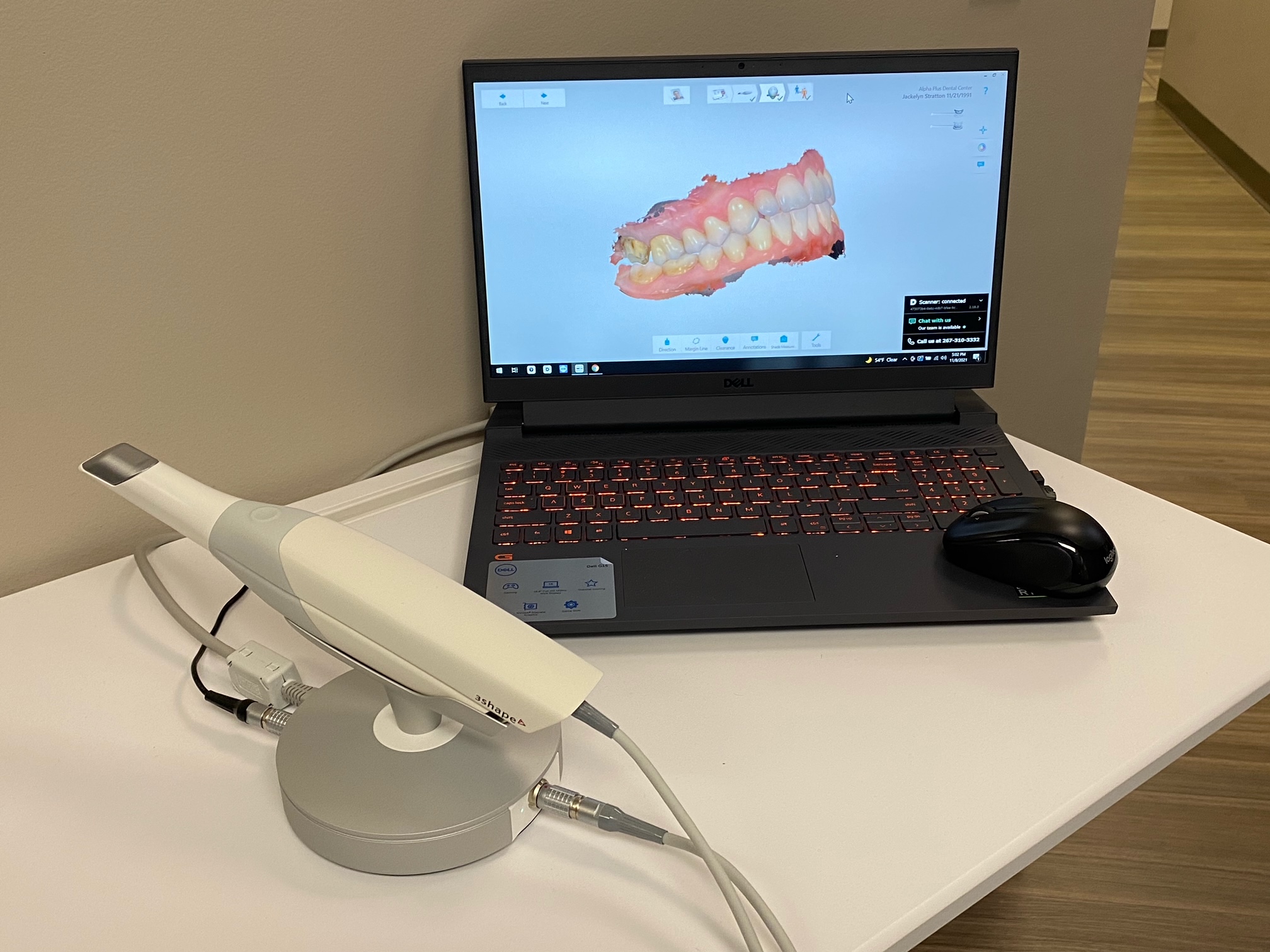
3Shape Intraoral Scanners: The Digital Cornerstone of Modern Dentistry
Intraoral scanners (IOS) have transitioned from luxury peripherals to non-negotiable clinical infrastructure in contemporary dental practice. The 3Shape TRIOS platform exemplifies this evolution, serving as the central nervous system for integrated digital workflows. Modern clinics deploying high-fidelity IOS solutions achieve 32% faster case completion (per 2025 EAO data), reduce remakes by 41%, and enable same-day restorations – critical advantages in value-based reimbursement models. Crucially, these systems generate the foundational digital datasets required for AI-driven treatment planning, CBCT fusion, and teledentistry integrations that define next-generation care delivery.
European manufacturers like 3Shape (Denmark) maintain leadership in clinical precision and ecosystem integration, with sub-10μm accuracy enabling complex prosthodontics and implant planning. However, this premium capability commands significant capital investment ($28,000-$42,000) and recurring software fees. Conversely, Chinese manufacturers including Carejoy have disrupted the mid-tier market with cost-optimized alternatives (sub-$15,000) targeting high-volume general practices. While functional for basic crown/bridge workflows, these systems often exhibit limitations in scan stability, material compatibility, and long-term service support – factors requiring rigorous ROI analysis beyond initial acquisition cost.
Strategic Equipment Comparison: Global Premium Brands vs. Value-Focused Chinese Manufacturers
The following analysis evaluates critical performance parameters for dental clinics evaluating scanner investments in 2026. European brands (represented by 3Shape TRIOS 5, Dentsply Sirona CEREC Omnicam, and Planmeca Emerald) are benchmarked against Carejoy’s flagship S900 series as the leading Chinese alternative. Note that “Global Brands” denotes established European/American manufacturers with ISO 13485-certified clinical validation.
| Technical Parameter | Global Brands (e.g., 3Shape TRIOS 5) | Carejoy S900 Series |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 (system + 1st-year software) | $8,900 – $14,500 (hardware-only) |
| Clinical Accuracy (μm) | 8.2 – 10.5 (ISO/IEC 17025 certified) | 18.3 – 24.7 (manufacturer-reported) |
| Full-Arch Scan Time | 62 – 78 seconds (continuous video capture) | 95 – 130 seconds (requires segmentation) |
| Material Compatibility | Full spectrum (including dark amalgam, wet surfaces, titanium) | Limited with dark/reflective materials; requires powder for bleeding patients |
| Software Ecosystem | Integrated CAD/CAM, AI diagnostics, lab communication hub, cloud DICOM | Basic CAD; requires third-party modules for lab integration ($2,200+) |
| Service Network | 24/7 regional support centers; 48-hr onsite SLA in 45+ countries | Centralized Asia support; 10-14 day turnaround for EMEA repairs |
| Warranty Structure | 3 years comprehensive (scanner + software) | 1 year limited hardware (excludes software updates) |
| Ideal Clinical Application | Full-scope digital dentistry: Implants, complex prosthetics, ortho, surgical guides | Routine crown/bridge; limited to simple single-unit restorations |
Strategic Recommendation: Premium European scanners remain indispensable for practices pursuing comprehensive digital integration and complex case acceptance. Carejoy represents a viable entry-point for budget-constrained clinics focusing exclusively on basic restorative work, though hidden costs of workflow interruptions and limited upgrade paths require careful modeling. Distributors should position Carejoy as a complementary solution for satellite clinics within multi-location groups, while reserving premium brands for flagship practices demanding clinical excellence. The 2026 market shift toward outcome-based dentistry will increasingly favor validated accuracy over acquisition cost – a critical consideration for forward-looking procurement strategies.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
3Shape Intraoral Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion battery, 3.7V, 3200 mAh; operating time up to 4 hours continuous scanning; charging time: ~2.5 hours via USB-C dock | Lithium-ion battery, 3.7V, 5200 mAh; operating time up to 7 hours continuous scanning; fast-charging support (0–80% in 60 mins) via proprietary docking station |
| Dimensions | 240 mm (L) × 28 mm (D) × 32 mm (W); scanner tip diameter: 10.5 mm; weight: 180 g (including handle) | 245 mm (L) × 26 mm (D) × 30 mm (W); scanner tip diameter: 9.0 mm; weight: 175 g (including ergonomic handle) |
| Precision | Accuracy: ≤ 20 μm; repeatability: ≤ 15 μm; scanning resolution: 1600 dpi; real-time 3D reconstruction at 24 fps | Accuracy: ≤ 12 μm; repeatability: ≤ 8 μm; scanning resolution: 2200 dpi; real-time 3D reconstruction at 32 fps with AI-powered edge detection |
| Material | Medical-grade polycarbonate housing; stainless steel scanning tip with anti-scratch sapphire lens; IP54 rated for dust and splash resistance | Carbon-fiber reinforced polymer body; titanium-coated scanning tip; sapphire lens with hydrophobic coating; IP55 rated with enhanced sterilization compatibility (autoclavable up to 134°C, 20 cycles) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS and REACH certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 10993-1 (biocompatibility), MDR 2017/745 compliant, GDPR-ready data handling |
Note: Specifications are based on 3Shape TRIOS 4 Standard and TRIOS 5 Advanced models as of Q1 2026. All values subject to change with firmware updates and regional regulatory requirements. Recommended for integration with 3Shape Dental System™ and third-party CAD/CAM platforms via open STL and DICOM export.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Why Source Intraoral Scanners from China in 2026?
China’s dental technology sector has matured significantly, with manufacturers now offering:
• Advanced optical engines (up to 1600 dpi resolution)
• AI-powered margin detection & shade matching
• Competitive TCO (Total Cost of Ownership) vs. premium European brands
• Flexible OEM/ODM capabilities for private labeling
• Shorter lead times (avg. 45-60 days post-PO vs. 90+ days for EU/US brands)
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 regulatory tightening requires multi-layered verification. Do not accept self-attested certificates.
| Verification Step | 2026 Compliance Requirement | Action Protocol |
|---|---|---|
| ISO 13485:2025 Certification | Must cover “Design & Manufacturing of Dental Imaging Devices” | Request certificate + scope page via official certification body portal (e.g., SGS, TÜV). Verify active status on IAF CertSearch |
| EU MDR (2017/745) Compliance | CE Mark under MDR (not legacy MDD) | Demand EC Certificate of Conformity showing MDR Annex IX route. Confirm Notified Body ID (e.g., 0123) is valid on EUDAMED |
| US FDA Establishment Registration | Required for US-bound shipments | Validate FERN number via FDA Device Registration & Listing Database |
| China NMPA Registration | Mandatory for all Chinese manufacturers | Check device listing on NMPA website (国药监械准字) |
Shanghai Carejoy: Verified Compliance Partner
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2025 Certificate #CN2026MD1874 (Issued by TÜV Rheinland)
- EU MDR CE Certificate #MDR-2026-CHN-0882 (Notified Body: DE-98-CE-2201)
- Active FDA Registration #301874521 (FERN: 1874521)
- NMPA Registration #国械注准20263060187
Verification Tip: Request Carejoy’s compliance dossier via [email protected] for independent validation. Their 19-year export history includes zero regulatory rejections across 87 countries.
2. Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics have shifted MOQ expectations. Key negotiation levers:
| MOQ Factor | 2025 Market Standard | 2026 Negotiation Strategy |
|---|---|---|
| White-Label Units | 50 units | Negotiate 20-30 units for distributors with 3+ year commitment. Carejoy offers 15-unit MOQ for first-time OEM clients with prepayment |
| Custom Firmware/UI | 100 units | Cap development fees at $8,500. Carejoy provides modular UI customization at 50-unit MOQ |
| Accessories Bundle | Fixed with scanner | Negotiate à la carte bundles (e.g., 100 tips per 5 scanners). Carejoy allows 30% accessory customization at 30-unit MOQ |
| Payment Terms | 30% deposit, 70% pre-shipment | Secure 50% LC at sight + 50% against B/L copy. Carejoy offers 15-day post-delivery terms for Tier-1 distributors |
3. Shipping Terms: DDP vs. FOB (2026 Critical Analysis)
Post-pandemic logistics require strategic freight term selection:
| Term | 2026 Risk Profile | When to Use | Cost Impact |
|---|---|---|---|
| FOB Shanghai | High (Customs clearance, inland freight, port delays) | For distributors with in-house logistics teams & Chinese customs brokers | Base freight + $380-520 DTHC (Destination Terminal Handling Charges) + 12-18% customs duty (varies by country) |
| DDP (Delivered Duty Paid) | Low (Supplier manages all risks) | Recommended for 95% of new buyers. Essential for clinics without import expertise | 18-25% premium over FOB but includes: • All freight & insurance • Duty/tax prepayment • Last-mile delivery to clinic • 2026 Advantage: Carbon-neutral shipping surcharge included |
2026 Best Practice: Insist on blockchain-tracked shipments (e.g., TradeLens) with real-time CO2 monitoring. Carejoy provides DDP quotes with verified carbon footprint data per ISO 14067.
Why Shanghai Carejoy Stands Out in 2026
Based on 19 years of specialized dental manufacturing (Baoshan District, Shanghai), Carejoy offers:
- Vertical Integration: In-house optical sensor production (reduces lead times by 30%)
- Regulatory Agility: Dedicated EU MDR/US FDA compliance team (avg. 22-day certification turnaround)
- Distributor Support: Co-branded marketing kits, multilingual technical training (including AR remote assistance)
- Quality Control: 100% pre-shipment calibration verification against ISO 10970:2025 standards
Engage Shanghai Carejoy for Verified Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1888 Jiangyang Road, Baoshan District, Shanghai 200430, China
Core Capability: Factory Direct OEM/ODM for Dental Scanners, Chairs, CBCT & Sterilization Systems
Contact:
• Email: [email protected]
• WhatsApp: +86 15951276160 (24/7 Technical Support)
• Verification Portal: compliance.carejoydental.com
Note: All technical specifications subject to 2026 regulatory updates. Request Carejoy’s Quarterly Compliance Bulletin (Q1 2026) for latest certification status.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: 3Shape TRIOS Intraoral Scanner – Purchasing Insights for 2026
Frequently Asked Questions (FAQ)
Below are key considerations for dental clinics and distribution partners evaluating the acquisition of 3Shape TRIOS intraoral scanners in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 3Shape TRIOS scanner (2026 model) support, and is it compatible with global power standards? | The 2026 3Shape TRIOS intraoral scanner operates on a universal power input of 100–240 V AC, 50/60 Hz, making it compatible with electrical systems across North America, Europe, Asia, and other global regions. The included power adapter automatically adjusts to local voltage, ensuring safe and reliable operation without the need for external transformers. Always verify local socket types and use appropriate plug adapters where necessary. |
| 2. Are spare parts for the 3Shape TRIOS scanner readily available, and how does 3Shape support long-term serviceability? | Yes, 3Shape maintains an extensive global spare parts network through authorized distributors and service centers. Common consumables (e.g., scan tips, sleeves, batteries) and critical components (e.g., scanner head, charging dock, LED modules) are stocked regionally to ensure rapid turnaround. 3Shape guarantees parts availability for a minimum of 7 years post-discontinuation of each scanner model, supporting long-term clinical uptime and investment protection. |
| 3. What does the installation process involve, and is on-site support provided for new 3Shape TRIOS systems? | Installation of the 3Shape TRIOS scanner includes hardware setup, software integration with 3Shape Communicate or your clinic’s existing dental software (e.g., exocad, CEREC), and network configuration. Authorized 3Shape partners provide remote or on-site installation services depending on regional agreements. On-site setup is standard for enterprise deployments and multi-unit clinic purchases. Training on calibration, scanning protocols, and maintenance is included as part of the onboarding package. |
| 4. What is the warranty coverage for the 3Shape TRIOS scanner purchased in 2026, and are extended service plans available? | All 3Shape TRIOS scanners purchased in 2026 come with a standard 2-year comprehensive warranty covering defects in materials and workmanship, including the scanner body, battery, and charging station. An optional 3Shape Service Plus extended warranty is available, extending coverage up to 5 years with benefits including priority repair, loaner unit availability during servicing, and preventive maintenance checks. Distributors can offer tailored service agreements for multi-unit clinics and corporate practices. |
| 5. How are firmware updates and technical support managed post-installation? | 3Shape provides over-the-air (OTA) firmware updates via the 3Shape Device Manager, ensuring scanners remain up-to-date with the latest scanning algorithms, material libraries, and software integrations. Technical support is accessible 24/7 through 3Shape’s global support portal and local distributor networks. Registered clinics receive proactive notifications for updates, service reminders, and cybersecurity patches to maintain compliance and optimal performance. |
Information accurate as of Q1 2026. Subject to change based on manufacturer specifications.
Need a Quote for 3Shape Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


