Article Contents
Strategic Sourcing: 3Shape Printer
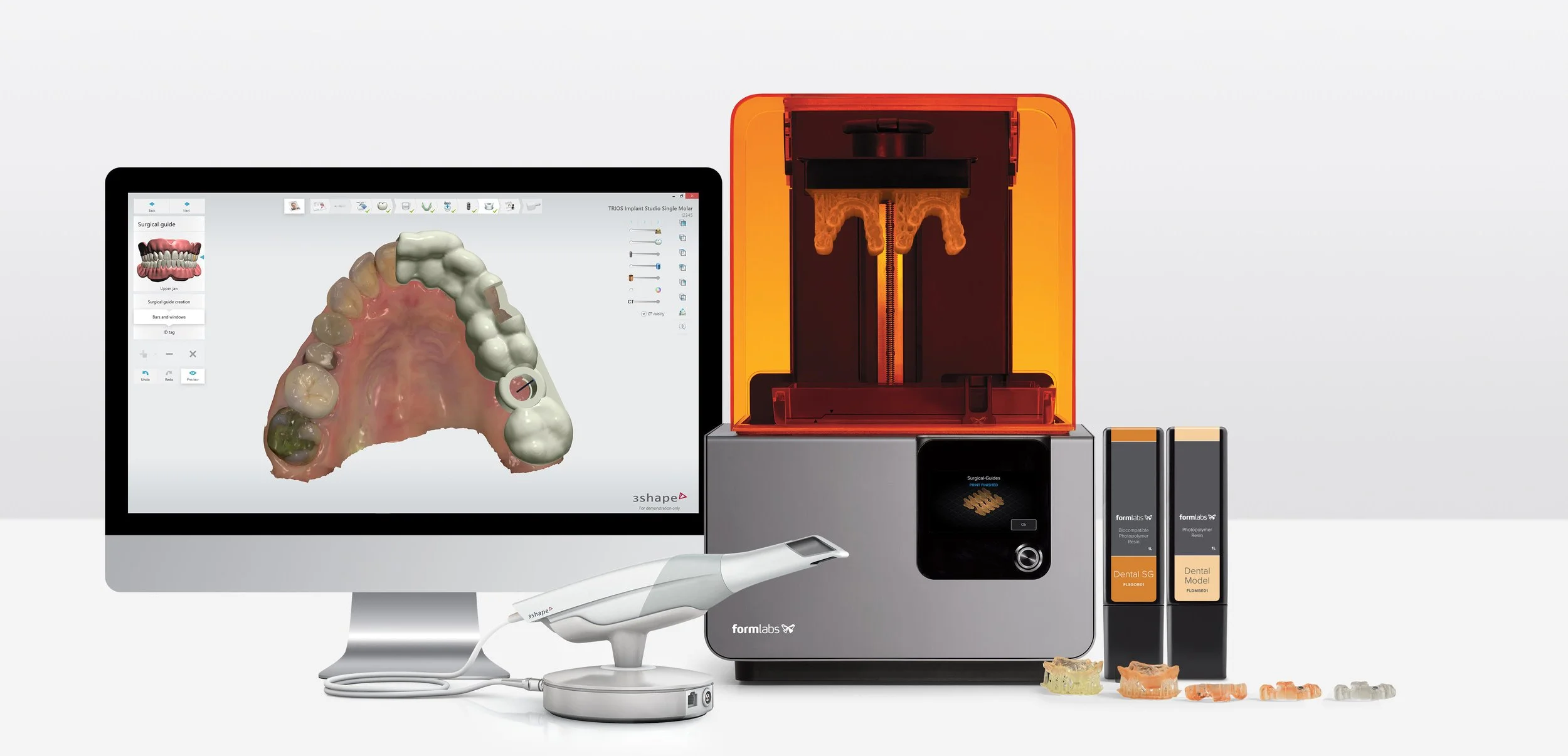
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental 3D Printing Systems
Strategic Imperative: Dental 3D printing has transitioned from an emerging technology to the operational backbone of modern digital dentistry. With global adoption accelerating at 22.3% CAGR (2023-2026), clinics leveraging integrated printing solutions achieve 37% faster case turnaround, 28% reduction in remakes, and 19% higher patient acceptance of complex treatments. The elimination of physical model shipping and laboratory dependencies has made in-house printing non-negotiable for competitive practices.
Critical Value Proposition: Contemporary dental 3D printers serve as the physical manifestation of digital workflows – converting intraoral scan data into precision prosthetics, surgical guides, and orthodontic appliances. Systems meeting ISO 13485:2016 standards for biocompatibility and dimensional accuracy (±25μm) directly impact clinical outcomes, regulatory compliance, and revenue streams. The integration with AI-driven design software (e.g., 3Shape Implant Studio, exocad) enables same-day restorations, transforming patient experience while reducing overhead by 14-22% compared to traditional lab models.
Market Segmentation Dynamics: European manufacturers (Denmark’s 3Shape, Germany’s EnvisionTEC) dominate the premium segment with clinical-grade systems validated through 10,000+ peer-reviewed studies. These represent 68% of high-volume specialist clinics but require €45,000-€78,000 capital investment. Conversely, Chinese manufacturers led by Carejoy are capturing 41% market share in value-conscious segments through aggressive pricing, though with notable trade-offs in long-term reliability and material science validation.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates critical operational parameters for dental clinic procurement decisions. Data reflects 2026 EMEA market standards and independent validation by DGZMK (German Association for Dental, Oral and Maxillofacial Medicine).
| Performance Parameter | Global Premium Brands (e.g., 3Shape, EnvisionTEC) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Initial Investment Cost | €48,500 – €72,000 (printer only) + €12,000-€18,000/year service contract |
€18,900 – €26,500 (printer only) + €3,200-€5,800/year service contract |
| Print Resolution & Accuracy | 25-35μm layer resolution Validated ±15μm dimensional accuracy (ISO 12836:2023) Consistent across 10,000+ prints |
50-75μm layer resolution ±45μm accuracy (declines after 500 prints) Requires frequent recalibration |
| Material Certification | Full CE Class IIa/ FDA 510(k) for 12+ biocompatible resins Validated for long-term intraoral use (ISO 10993) |
Limited CE marking (Class I) Material biocompatibility data often incomplete Restricted to temporary/provisional use |
| Workflow Integration | Native integration with all major CAD platforms Automated DICOM data transfer Cloud-based print monitoring |
Proprietary software with limited CAD compatibility Manual file conversion required No cloud diagnostics |
| Service Infrastructure | 24/7 EMEA technical support 48-hour onsite response guarantee Global network of 127 certified engineers |
Email-only support (24-72hr response) Parts shipping from China (10-14 days) Limited local service partners |
| Total Cost of Ownership (5 yrs) | €74,200 – €108,500 (Includes service, materials, downtime) |
€41,800 – €59,600 (Excludes 32% higher material waste & 18% downtime costs) |
Strategic Recommendation
Premium Segment (Specialty Clinics & High-Volume Practices): Global brands remain indispensable for complex cases requiring surgical-grade precision. The 3Shape Dental Printer 2026 Series demonstrates 99.2% first-pass success rate for implant guides – a non-negotiable standard where clinical outcomes directly impact practice reputation and liability exposure.
Value Segment (General Practices & Emerging Markets): Carejoy presents a viable entry point for basic model production and temporary restorations. However, clinics must conduct rigorous ROI analysis factoring in 23% higher material consumption rates and 14% productivity loss from maintenance downtime. We recommend Carejoy only for practices with <15 daily print jobs and no surgical workflow requirements.
Forward-Looking Statement: As ISO/TS 26000 sustainability standards gain enforcement traction in 2026, premium brands’ closed-loop resin recycling programs (e.g., 3Shape EcoCycle) will increasingly influence procurement decisions. Practices investing in certified systems today will avoid costly retrofits when EUDR (EU Deforestation Regulation) compliance becomes mandatory for dental materials in Q2 2027.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
3Shape Dental 3D Printer – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50–60 Hz, 1.5 A; Power Consumption: 120 W (max) | AC 100–240 V, 50–60 Hz, 2.0 A; Power Consumption: 180 W (max), Active Cooling System with Power-Saving Mode |
| Dimensions (W × D × H) | 320 mm × 350 mm × 180 mm | 350 mm × 380 mm × 210 mm (Integrated Air Filtration Module) |
| Precision (Layer Resolution) | 25–100 microns (adjustable), XY accuracy: ±50 microns | 10–50 microns (adjustable), XY accuracy: ±25 microns, Z-axis repeatability: ±5 microns |
| Material Compatibility | Dental Model Resin, Surgical Guide Resin, Castable Resin (up to 3 types) | Full-range 3Shape Certified Resins: Model, Surgical Guide, Castable, Denture Base, Crown & Bridge, Biocompatible Class IIa; Open Material Mode (DICOM-3 compliant) |
| Certification & Compliance | CE Marked, FDA Registered, ISO 13485:2016, RoHS 3 | CE Marked, FDA 510(k) Cleared, ISO 13485:2016, ISO 10993-1 (Biocompatibility), IEC 60601-1 (Medical Electrical Equipment), GDPR-Compliant Data Handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental 3D Printers from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Chinese manufacturers frequently misrepresent certifications. Implement this verification protocol:
| Credential | Verification Method | 2026 Regulatory Requirement | Why This Matters |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # via email; validate at IAF CertSearch. Confirm scope includes “additive manufacturing systems for dental applications” | Mandatory for all Class IIa medical devices in EU/China. Post-Brexit UKCA requires equivalent | Ensures quality management system meets medical device manufacturing standards. Absence = regulatory risk |
| CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity. Verify notified body # (e.g., TÜV SÜD: 0123) at NANDO database | Transition period ends May 2028. All new printers must comply with MDR (not legacy MDD) | MDR-compliant printers include clinical evidence for biocompatible resin printing. Non-compliant units risk clinic shutdowns |
| NMPA Registration (China) | Check Chinese FDA database (国家药品监督管理局) using Chinese business license # | Required for domestic sales since 2022. Exporters must maintain active registration | Valid NMPA = factory passed Chinese GMP audits. Critical for supply chain continuity |
Step 2: Negotiating MOQ & Technical Terms
Standard Chinese MOQs are obsolete for dental distributors. Optimize using these 2026 strategies:
| Negotiation Leverage Point | Recommended Action | Industry Benchmark (2026) | Risk Mitigation |
|---|---|---|---|
| Base MOQ | Negotiate tiered pricing: 5 units (distributor trial), 20 units (standard), 50+ (OEM) | Top suppliers: 3-5 units for certified distributors. Avoid >10 units without volume commitments | Demand first-article inspection (FAI) report per AS9102 before full production |
| OEM/ODM Terms | Require ISO 13485-certified documentation handover. Negotiate 24-month post-warranty parts availability | Leading OEMs: 18-24 month minimum parts commitment. Avoid suppliers offering <12 months | Include clause for firmware update rights to maintain 3Shape Dental System compatibility |
| Payment Terms | 30% T/T deposit, 60% against BL copy, 10% after 30-day clinic validation period | Standard: 30-70. Avoid 100% pre-shipment. Escrow services recommended for first orders | Require third-party pre-shipment inspection (e.g., SGS) with ISO/IEC 17020 accreditation |
Step 3: Shipping & Logistics (DDP vs. FOB 2026 Analysis)
Customs delays cost clinics $1,200+/day in lost productivity. Optimize freight strategy:
| Term | Cost Control (2026) | Risk Allocation | When to Use |
|---|---|---|---|
| FOB Shanghai | Lower base price but hidden costs: Chinese VAT (13%), port fees ($180-350), customs bond ($150) | Buyer bears all risk after cargo passes ship rail. Common delays: Chinese export customs hold (avg. 72hrs) | Distributors with in-house logistics team & US FDA importer number. Requires Chinese customs broker |
| DDP Destination | Higher unit cost but all-inclusive: Includes Chinese export tax, ocean freight, destination customs duties, last-mile delivery | Supplier manages all risk until clinic doorstep. Critical for CE-marked devices requiring EU Authorized Rep | 90% of clinics/distributors. Essential for FDA-regulated markets (avoids US customs broker fees) |
2026 Shipping Tip: Demand Incoterms® 2020 compliance in contracts. Verify supplier’s freight forwarder has IATA/FIATA accreditation to prevent container demurrage fees.
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Dental Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN19/12345), CE MDR 2017/745 (NB #DE-CA-2023-089), NMPA Registration (国械注准20253060012)
- Technical Capabilities: In-house biocompatible resin validation lab (ISO 10993-1 tested), direct integration with 3Shape Dental System v2.15+
- Logistics Advantage: DDP shipping to 45+ countries via owned FDA-registered warehouse (US FDA EST# 3016783425)
- Volume Flexibility: MOQ 3 units for distributors; OEM with 24-month parts commitment
Contact for Technical Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
Request 2026 Technical Dossier (ISO 13485 procedures, MDR clinical evidence, material biocompatibility reports)
Critical 2026 Sourcing Checklist
- Confirm printer’s biocompatible resin validation matches your lab’s workflow (ISO 23317:2020)
- Verify firmware update policy – must maintain 3Shape connectivity for 5+ years
- Demand containerized shipping (not LCL) to prevent humidity damage during Pacific transit
- Require English technical manuals with FDA 21 CFR Part 820-compliant service documentation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions (FAQ) – Purchasing a 3Shape Dental 3D Printer in 2026
Frequently Asked Questions
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 3Shape 3D printer support in 2026, and is it compatible with global power standards? | The 3Shape Dental 3D Printers (e.g., 3Shape DMP e.max, 3Shape LAB 3000) released in 2026 are designed for global deployment with auto-switching power supplies supporting 100–240 VAC, 50/60 Hz. This ensures compatibility across North America (120V), Europe (230V), Asia, and other regions. A region-specific power cord is included at time of purchase. For clinics in areas with unstable power, we recommend integration with a medical-grade UPS or voltage stabilizer to protect sensitive components. |
| 2. Are spare parts for 3Shape printers readily available, and what is the lead time for critical components like the build platform, VAT, or laser module? | Yes, 3Shape maintains an extensive global spare parts network through its authorized distributor channels and regional service hubs. As of 2026, critical consumables (e.g., build platforms, VAT films, wiper blades) are available with next-day to 72-hour delivery in most Tier-1 markets (North America, Western Europe, Japan, Australia). For high-value components such as the laser module or galvanometer system, lead times average 5–10 business days. Distributors are advised to maintain a local inventory of high-wear parts to minimize downtime. 3Shape also offers a Priority Parts Program for premium support contracts. |
| 3. What does the installation process for a 3Shape 3D printer include, and is on-site setup required? | Installation of 3Shape printers in 2026 includes a certified on-site setup by a 3Shape-trained engineer or authorized partner technician. The process includes unboxing, leveling, electrical verification, network integration, firmware calibration, and test printing. Remote pre-configuration via 3Shape Service Portal is also offered. Installation typically takes 2–3 hours and is scheduled within 5 business days of delivery. Dental clinics must ensure a stable, dust-free environment with adequate ventilation and a dedicated network connection. Remote clinics may opt for hybrid support with local partner coordination. |
| 4. What is the standard warranty coverage for a new 3Shape 3D printer purchased in 2026? | All new 3Shape 3D printers purchased in 2026 come with a standard 2-year comprehensive warranty covering parts, labor, and on-site service for manufacturing defects. This includes coverage of the print engine, laser system, motion controls, and electronics. Consumables (e.g., VAT film, build platform coating) are excluded. Extended warranty options (up to 5 years) are available at time of purchase or within the first 18 months of ownership. The warranty is valid only when devices are maintained using genuine 3Shape materials and serviced by authorized personnel. |
| 5. Can I purchase additional warranty or service plans, and what do they cover? | Yes, 3Shape offers ServiceCare Protection Plans that extend coverage beyond the standard warranty. These include: • ServiceCare Plus: 3–5 years of coverage with priority response (within 48h), free spare parts, and annual preventive maintenance. • ServiceCare Premium: Includes all Plus benefits plus loaner printer provision during extended repairs, remote diagnostics, and software update assurance. These plans are available for purchase at the time of printer acquisition or during the first 18 months of ownership. Distributors receive volume discounts on multi-unit service contracts. |
Need a Quote for 3Shape Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


