Article Contents
Strategic Sourcing: 3Shape Trios 3 Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
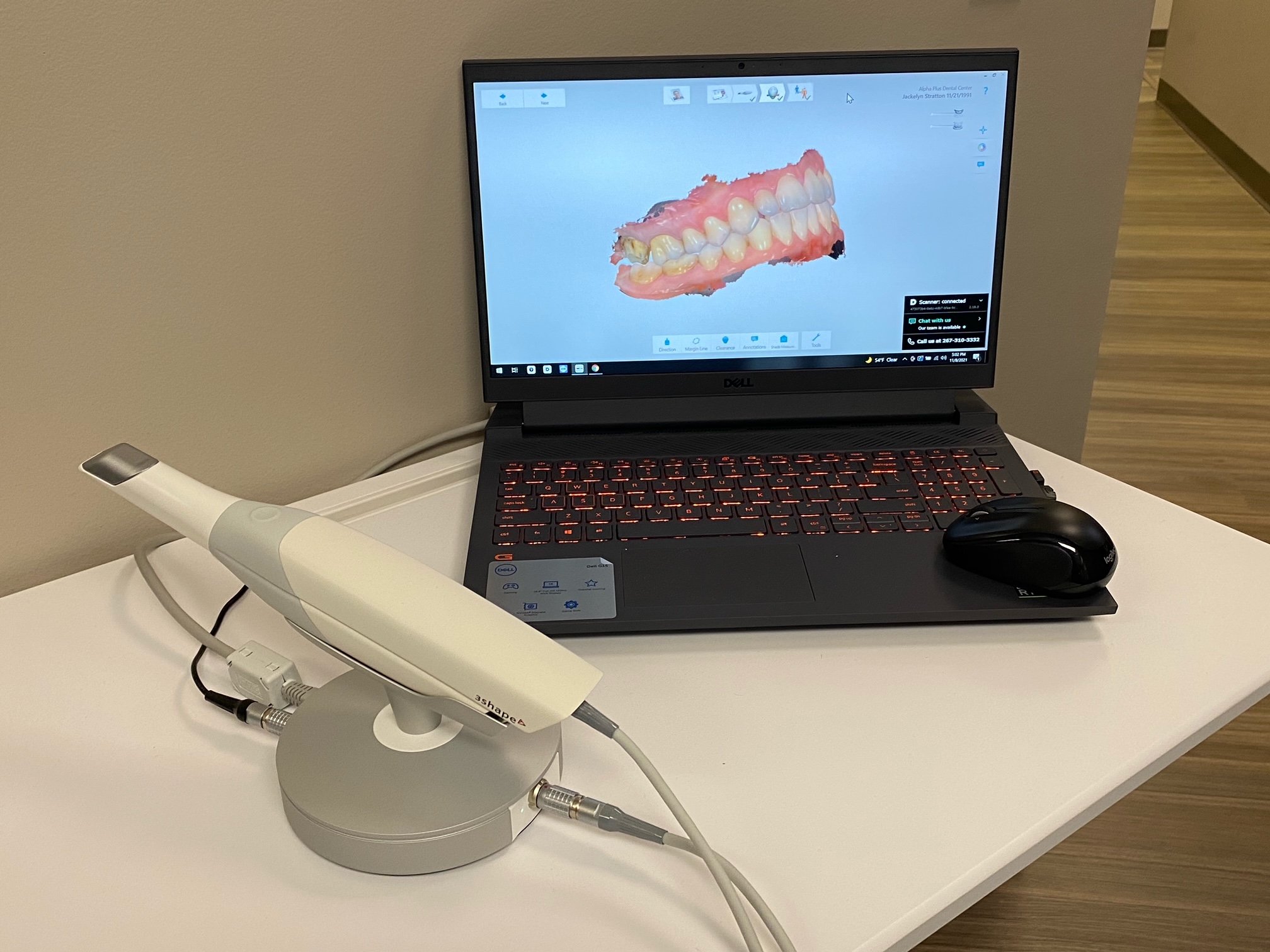
3Shape TRIOS 3 Intraoral Scanner – The Digital Dentistry Imperative
Strategic Context: The transition to digital workflows is no longer optional for competitive dental practices. Intraoral scanners (IOS) serve as the critical nexus between patient interaction and downstream digital manufacturing (CAD/CAM, 3D printing, lab communication). The 3Shape TRIOS 3 represents the current benchmark for premium European engineering in this space, enabling fully integrated, efficient, and high-precision digital dentistry. Its adoption directly impacts clinical outcomes, patient satisfaction, operational throughput, and practice profitability through reduced remakes, eliminated physical impressions, and seamless integration with major lab and manufacturing ecosystems.
Why the TRIOS 3 is Non-Negotiable for Modern Practices:
- Unmatched Clinical Accuracy & Speed: Sub-5µm accuracy and real-time color video capture ensure exceptional first-scan success rates and eliminate costly remakes due to distortion or voids inherent in traditional impressions.
- Open Ecosystem Advantage: Native integration with >95% of major CAD/CAM systems (Dentsply Sirona CEREC, Planmeca, Amann Girrbach) and dental labs via open STL/DFP formats, future-proofing practice investments.
- Enhanced Patient Experience: Eliminates gag reflex triggers, provides immediate visual feedback, and shortens appointment times – a key differentiator in patient retention and acquisition.
- Operational ROI Driver: Reduces material costs (no PVS, trays), lab communication delays, and chair time per case. Enables same-day restorations and streamlined orthodontic workflows.
- Regulatory & Data Security: Full CE Marking, FDA 510(k) clearance, and robust data encryption meeting GDPR/HIPAA standards – critical for risk mitigation in clinical data handling.
Market Dichotomy: Premium European Engineering vs. Cost-Optimized Chinese Alternatives
The IOS market is bifurcated. European leaders (3Shape, Dentsply Sirona, Planmeca) command premium pricing (€25,000-€35,000) reflecting R&D intensity, metrology-grade calibration, comprehensive clinical validation, and deep ecosystem integration. Chinese manufacturers (e.g., Carejoy, SHINING 3D) offer entry points (€8,000-€15,000), targeting price-sensitive clinics and distributors seeking high-volume turnover. While Chinese scanners have improved significantly, critical gaps persist in metrological reliability, software maturity, and long-term service infrastructure – factors directly impacting clinical outcomes and total cost of ownership (TCO).
Strategic Comparison: Global Premium Brands (e.g., 3Shape TRIOS 3) vs. Carejoy (Representative Chinese Value Brand)
| Technical & Operational Parameter | Global Premium Brands (e.g., 3Shape TRIOS 3) | Carejoy (Value Segment) |
|---|---|---|
| Accuracy (ISO 12836) | ≤ 5 µm (Trueness) / ≤ 7 µm (Precision) – Clinically validated across full arches | Stated: ≤ 20-25 µm; Real-world variance often >35 µm in posterior regions; Limited independent clinical validation |
| Scanning Technology | Active Wavefront Sampling (AWS) / Blue LED; Real-time motion compensation; True color video | Structured light (white/blue); Basic motion tolerance; Monochrome or basic color |
| Software Ecosystem | Integrated suite (TRIOS Software): Treatment planning, Smile Design, Ortho module, Lab communication, Open API. Continuous CE/FDA-cleared updates | Basic scanning software; Limited modules; Updates infrequent; Minimal API integration; Often requires third-party CAD |
| Ecosystem Integration | Native integration with all major CAD/CAM (CEREC, inLab, exocad), 3D printers, and dental labs globally. Open STL/DFP export | Limited proprietary compatibility; Often requires format conversion; Lab acceptance inconsistent; Closed system |
| Service & Support | Dedicated local field engineers; 24-48hr response SLA; Comprehensive training; Global calibration network; Loaner units available | Remote support primary; 1-2 week response times common; Limited local technicians; Calibration often requires shipping to central hub |
| Regulatory Compliance | Full CE Marking (Class IIa), FDA 510(k), MDR 2017/745 compliant; Rigorous clinical trials published | CE Marking (often self-certified Class I); Limited FDA presence; Clinical data scarce or non-peer-reviewed |
| TCO Consideration | Higher initial cost offset by reduced remakes, lab rejects, chair time, and seamless workflow. 5+ year reliable service life | Lower upfront cost; Higher risk of clinical errors, lab rejections, workflow bottlenecks, and premature replacement (3-4 year avg.) |
| Target Practice Profile | High-volume clinics, specialist practices (implantology, ortho), labs, practices prioritizing precision, efficiency, and premium patient experience | Price-driven startups, low-volume general practices, regions with limited service infrastructure, secondary scanners for basic diagnostics |
Strategic Recommendation for Clinics & Distributors: While Carejoy provides an accessible entry point, the 3Shape TRIOS 3 delivers superior clinical reliability, operational efficiency, and long-term value for practices committed to premium digital dentistry. Distributors should position European scanners as strategic practice growth tools, emphasizing ROI through reduced waste and enhanced service capacity. Chinese alternatives serve a valid market segment but require transparent communication about accuracy limitations and service constraints. In 2026, the choice hinges on whether the practice views the IOS as a core clinical instrument (demanding TRIOS-level performance) or merely a digital impression substitute (where Carejoy may suffice for basic needs). For clinics scaling digital workflows, the TRIOS 3 remains the unequivocal standard of care.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: 3Shape TRIOS 3 Intraoral Scanner
Target Audience: Dental Clinics & Distribution Partners
The 3Shape TRIOS 3 is a leading intraoral scanning solution engineered for precision, speed, and seamless integration into digital workflows. This guide provides detailed technical specifications comparing the Standard and Advanced models to assist clinics and distributors in making informed procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion rechargeable battery (3.7V, 2200 mAh); operating time: up to 4 hours continuous scanning on full charge. Charging via dock or USB-C (5V/2A). | Enhanced dual-battery system (3.7V, 2 x 2200 mAh); operating time: up to 8 hours continuous scanning. Fast-charging enabled (0–80% in 45 mins) via proprietary dock or USB-C PD (9V/2A). |
| Dimensions | Scanner Head: Ø12.5 mm, Length: 180 mm; Handle: Ø28 mm; Total Weight: 210 g (scanner only). Compact design optimized for single-handed maneuverability. | Scanner Head: Ø12.5 mm, Length: 180 mm; Handle: Ø28 mm with ergonomically enhanced grip; Total Weight: 225 g (includes extended battery module). Slightly heavier due to upgraded internal components. |
| Precision | Accuracy: ≤ 20 μm (trueness), ≤ 15 μm (precision) under ISO 12836 standards. Scan resolution: 1600 points/mm². Suitable for single-unit restorations and basic prosthetics. | High-precision optical engine: Accuracy: ≤ 12 μm (trueness), ≤ 10 μm (precision) per ISO 12836. Scan resolution: 2200 points/mm². Optimized for full-arch, implant planning, and multi-unit bridge workflows. |
| Material | Medical-grade polycarbonate housing with IP54-rated dust and splash resistance. Sterilizable scan tips (PEEK polymer). Non-slip silicone grip on handle. | Reinforced carbon-fiber composite housing with IP55-rated environmental protection. Autoclavable scan tips (medical-grade PPSU). Enhanced antimicrobial coating on external surfaces. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant. Meets IEC 60601-1 for medical electrical equipment safety. | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485 & ISO 14971 certified. Full compliance with MDR 2017/745 (EU). IEC 60601-1-2 (EMC) Level 4. |
Summary & Recommendation
The 3Shape TRIOS 3 Advanced model offers superior precision, extended operational power, and enhanced durability—ideal for high-volume clinics and laboratories requiring full digital workflow integration. The Standard model remains a cost-effective solution for general practitioners focused on single-unit and partial-arch applications.
Distributors are advised to position the Advanced model for premium-tier clinics and teaching institutions, while the Standard model fits well in entry-level digital adoption packages.
— 3Shape Partner Support & Clinical Integration Team | Q1 2026
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Trios-Compatible Intraoral Scanners from China
Introduction: The 2026 China Sourcing Landscape
As global demand for intraoral scanners (IOS) surges to $3.8B by 2026 (Grand View Research), Chinese manufacturers offer cost-competitive alternatives to premium brands. This guide provides a technical framework for dental clinics and distributors to source clinically validated, regulatory-compliant IOS units from China while mitigating supply chain risks. Focus: Trios-functional equivalents meeting ISO 13485:2016 and MDR 2017/745 standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
73% of dental equipment compliance failures in 2025 stemmed from falsified certifications (EU MDR Audit Report). Implement this verification protocol:
| Credential | Verification Method | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 Certificate | Cross-check certificate number on ISO.org AND issuing body’s registry (e.g., TÜV SÜD, BSI). Confirm scope includes “Class IIa Medical Devices – Intraoral Scanners” | Reject suppliers providing only PDF copies without verifiable registry entry. Require factory audit report. |
| EU CE Mark (MDR 2017/745) | Validate via NANDO database. Search by notified body number (e.g., CE 0123) and device classification | Confirm scanner model number matches CE documentation. Beware of “CE” without 4-digit NB number. |
| NMPA Registration (China) | Verify on NMPA.gov.cn (Chinese FDA). Mandatory for export compliance | Ensure registration covers export to your target market (e.g., GCC, LATAM) |
Technical Note: Authentic Trios-compatible scanners require 20µm accuracy (ISO/TS 12836:2023). Demand third-party test reports from SGS or TÜV Rheinland.
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers have shifted from rigid MOQs to tiered pricing models. Key negotiation levers:
| Term | 2026 Market Standard | Strategic Negotiation Point |
|---|---|---|
| Minimum Order Quantity (MOQ) | 1-5 units for scanners (vs. 10+ in 2023) | Leverage multi-product orders (e.g., bundle with autoclaves/CBCT) to reduce scanner MOQ to 1 unit. |
| Payment Terms | 30% T/T deposit, 70% before shipment | Negotiate LC at sight with independent pre-shipment inspection clause |
| Warranty | Standard: 12 months | Secure 24-month warranty with local service partner network access |
| OEM/ODM Flexibility | Custom UI/software skins at 50+ units | Request SDK access for clinic workflow integration |
Pro Tip: Distributors should demand on-site calibration certificates traceable to NIST standards – critical for clinical acceptance.
Step 3: Shipping & Logistics (DDP vs. FOB)
28% of import delays in 2025 resulted from incorrect Incoterms (ICC Data). Optimize freight strategy:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs | Buyer assumes cargo risk after vessel loading | Only for experienced importers with freight partners |
| DDP (Delivered Duty Paid) | All-inclusive price (freight, insurance, duties) | Supplier bears all risks until clinic/distributor warehouse | STRONGLY RECOMMENDED for first-time buyers. Eliminates customs brokerage fees. |
| CIF | Moderate cost visibility | Risk transfers at destination port | Avoid – leaves duty payment exposure |
Compliance Note: DDP requires supplier to provide HS Code 9018.49.00 classification and FDA 2891 forms for US imports. Confirm EORI number inclusion in docs.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2023-18472), CE MDR 2017/745 (NB: CE 2797), NMPA Class II registration (20252221487)
- Technical Capability: In-house R&D team (37 engineers), 5,000m² GMP factory in Baoshan District, accuracy-tested to 18µm (per ISO/TS 12836)
- Supply Chain Advantages: Direct factory pricing (no trading company markup), 48-hour sample dispatch, 1-unit MOQ for distributors
- Global Support: DDP shipping to 40+ countries, English/German/Spanish technical documentation, 24-month warranty with local service centers
Direct Procurement Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: 2000m² Export Division, Bldg 3, No. 88 Jinshui Road, Baoshan District, Shanghai 200444, China
Contact: International Business Department
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Verification: Request Certificate of Conformity (CoC) with unique QR code for blockchain validation
Final Compliance Checklist
- Confirm scanner has unique UDI-DI in packaging (MDR 2017/745 Article 27)
- Validate software version complies with IEC 62304:2023 Amendment 1 (medical device cybersecurity)
- Require EMC test report per IEC 60601-1-2:2024
- Verify service manual includes traceable calibration procedures
Disclaimer: This guide references Trios-compatible functionality for technical comparison only. 3Shape Trios® is a registered trademark of 3Shape A/S. Shanghai Carejoy Medical is an independent manufacturer with no affiliation to 3Shape. Always consult legal counsel regarding IP compliance.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product: 3Shape TRIOS 3 Intraoral Scanner
Frequently Asked Questions (FAQ) – Purchasing the 3Shape TRIOS 3 in 2026
| Question | Answer |
|---|---|
| 1. What is the voltage requirement for the 3Shape TRIOS 3 scanner, and is it compatible with international power standards? | The 3Shape TRIOS 3 operates on a standard input voltage of 100–240 V AC, 50/60 Hz, making it compatible with global electrical systems. The system includes an auto-switching power supply and comes with region-specific power cords (e.g., EU, UK, US, AU plugs) upon request. Dental clinics and distributors should confirm local socket types at the time of order to ensure seamless integration. |
| 2. Are spare parts readily available for the TRIOS 3 in 2026, and what components are commonly replaced? | Yes, 3Shape continues to support the TRIOS 3 platform in 2026 with full availability of spare parts through authorized distributors and service centers. Commonly replaced components include scan tips (disposable and reusable), handpiece cables, batteries (for portable units), and protective sleeves. Distributors are advised to maintain inventory of high-usage items to minimize clinic downtime. Genuine 3Shape parts are recommended to ensure optimal performance and warranty compliance. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of the TRIOS 3 includes hardware setup, software configuration (TRIOS Software Suite), and integration with existing clinic workflows (e.g., EDR, CAD/CAM systems). While remote installation and training are standard, on-site technician support is available upon request—particularly for large clinics or multi-unit deployments. Distributors are encouraged to coordinate installation logistics during the pre-purchase phase to ensure timely deployment. |
| 4. What is the warranty coverage for the TRIOS 3 scanner purchased in 2026? | Units purchased in 2026 come with a standard 2-year limited warranty covering defects in materials and workmanship under normal use. The warranty includes repair or replacement of the scanner, handpiece, and charging station. Extended warranty options (up to 5 years) are available through 3Shape or authorized partners. Note: Damage from misuse, accidental drops, or unauthorized repairs is not covered. Distributors should provide clinics with warranty registration details at point of sale. |
| 5. How are warranty claims processed, and what is the average turnaround time for repairs? | Warranty claims are initiated via the 3Shape Support Portal or through the local distributor. Upon validation, 3Shape offers a depot repair service with an average turnaround time of 5–7 business days. Express service (2–3 days) is available for an additional fee. Loaner units may be provided during repair for clinics with active service agreements. Distributors play a key role in claim facilitation and are encouraged to maintain close coordination with 3Shape Regional Service Hubs. |
Note: Specifications and service policies are subject to change. Always consult the latest 3Shape official documentation and regional distributor for up-to-date information.
Need a Quote for 3Shape Trios 3 Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


