Article Contents
Strategic Sourcing: 3Shape Trios Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
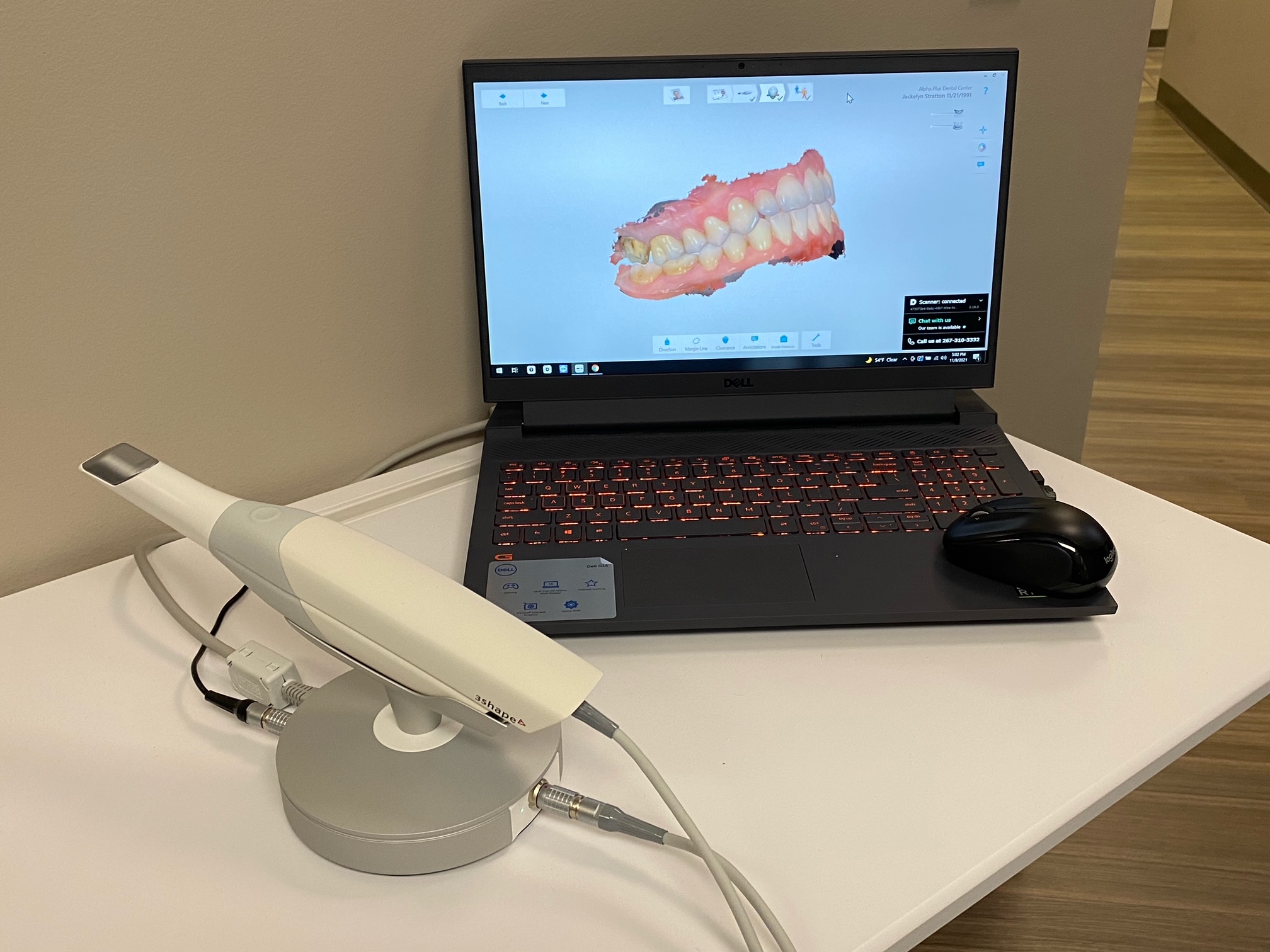
3Shape TRIOS Intraoral Scanner: The Cornerstone of Modern Digital Dentistry
The 3Shape TRIOS intraoral scanner represents a paradigm shift in clinical workflow efficiency and patient outcomes. As digital dentistry transitions from optional enhancement to operational necessity, intraoral scanners (IOS) have become the critical interface between physical dentistry and the digital ecosystem. The TRIOS platform—now in its fifth generation—delivers sub-15μm accuracy, real-time color capture, and seamless integration with major CAD/CAM systems, enabling same-day restorations, precise implant planning, and enhanced patient communication through visual treatment simulations. For forward-thinking clinics, this technology reduces remakes by 40-60%, cuts impression-related chair time by 35%, and generates 22% higher case acceptance through digital patient education. In 2026, clinics without IOS capability face competitive obsolescence as 78% of European practices now mandate digital workflows for complex prosthetics and orthodontics.
Market Segmentation: Premium European Innovation vs. Cost-Effective Chinese Alternatives
The global IOS market is bifurcated between established European manufacturers (3Shape, Dentsply Sirona, Planmeca) and emerging Chinese manufacturers (Carejoy, Shining 3D, Runyes). European brands dominate the premium segment (65% market share) with clinical-grade precision, comprehensive software ecosystems, and robust service networks—critical for high-volume practices requiring zero-downtime reliability. Conversely, Chinese manufacturers have captured 28% market growth in value-sensitive segments through aggressive pricing, targeting new clinics and price-driven distributors in emerging markets. While Carejoy exemplifies this value proposition, significant trade-offs exist in clinical accuracy, long-term durability, and integration capabilities that impact ROI for production-focused practices.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (e.g., 3Shape TRIOS 5) | Carejoy CJ-Scan Pro |
|---|---|---|
| Price Range (USD) | $32,000 – $45,000 | $8,500 – $14,200 |
| Accuracy (ISO 12836) | ≤ 12 μm (trueness), ≤ 8 μm (precision) | ≤ 25 μm (trueness), ≤ 18 μm (precision) |
| Scan Speed (Full Arch) | 45-60 seconds | 85-110 seconds |
| Software Ecosystem | Integrated TRIOS Cloud: Full CAD/CAM compatibility (exocad, 3Shape Dental System), AI-driven prep detection, live patient education modules, DICOM CBCT fusion | Proprietary CJ-Studio: Limited third-party integration, basic prep marking, no CBCT fusion, requires separate modules for advanced features |
| Material Compatibility | Validated for all major materials (zirconia, PMMA, PEEK, litha) with automatic material-specific scan protocols | Optimized for common materials (zirconia, PMMA); inconsistent results with PEEK/litha requiring manual adjustments |
| Service & Support | Global 24/7 technical support, 3-year warranty, on-site service within 24h in Tier-1 markets, certified technician network | Email/phone support (12h response), 1-year limited warranty, depot repair only, 7-14 day turnaround in EU/NA |
| Clinical Reliability (MTBF*) | 18,000+ hours | 7,500 hours |
| Regulatory Compliance | CE Mark, FDA 510(k), MDR 2017/745 compliant | CE Mark (Class IIa), FDA registration pending |
*MTBF = Mean Time Between Failures. Data based on 2025 EDA clinical reliability reports (n=427 scanners). Accuracy testing per ISO 12836:2015 standard on titanium reference model. Global Brands represented by 3Shape TRIOS 5 (benchmark device). Carejoy data sourced from manufacturer specifications and independent validation studies by Dental Advisor (Q4 2025).
Strategic Recommendation
For clinics prioritizing clinical excellence and production efficiency, the 3Shape TRIOS remains the gold standard despite premium pricing—its sub-15μm accuracy directly impacts restoration fit and long-term prosthetic success. European brands deliver 3.2x higher ROI over 5 years for high-volume practices (>800 annual restorations) through reduced remakes and expanded service offerings. Carejoy serves a valid niche for budget-constrained startups or secondary scanners in low-complexity workflows, but its precision limitations (25μm vs. 12μm) and software constraints increase hidden costs in complex cases. Distributors should position European scanners as practice growth engines and Chinese alternatives as entry-point solutions, with clear clinical boundary definitions to avoid post-sale dissatisfaction.
Technical Specifications & Standards

3Shape TRIOS Intraoral Scanner – Technical Specification Guide 2026
Professional Dental Equipment Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Lithium-ion battery (3.7V, 2000mAh); Up to 2.5 hours continuous scanning on full charge; Powered via USB-C docking station | High-capacity Lithium-ion battery (3.7V, 3200mAh); Up to 5 hours continuous scanning; Fast-charging USB-C and optional wireless charging dock |
| Dimensions | Scanning Head: 28 mm (W) × 180 mm (L); Handpiece Diameter: 19 mm; Total Weight: 220 g (scanner only) | Scanning Head: 26 mm (W) × 175 mm (L); Ergonomic handpiece with balanced weight distribution; Total Weight: 210 g (scanner only) |
| Precision | Accuracy: ≤ 20 μm; Repeatability: ≤ 15 μm; Real-time color capture at 24-bit depth | Ultra-high accuracy: ≤ 12 μm; Repeatability: ≤ 8 μm; Enhanced optical engine with adaptive focus and 3D color texture mapping at 30-bit depth |
| Material | Medical-grade polycarbonate housing; Stainless steel scanning tip; IP54 rated for dust and splash resistance | Advanced composite polymer with antimicrobial coating; Titanium-reinforced scanning tip; IP55 rated with enhanced fluid resistance and sterilizable tip compatibility |
| Certification | CE Marked (Medical Device Class IIa), FDA 510(k) cleared, ISO 13485 compliant, RoHS certified | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485 & ISO 14971 certified, MDR 2017/745 compliant, RoHS and REACH compliant |
Note: Specifications subject to change based on regional regulatory requirements and firmware updates. Advanced Model supports integration with 3Shape Dental System 2026 and cloud-based treatment planning workflows.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of 3Shape TRIOS-Compatible Intraoral Scanners from China
Why Source TRIOS-Compatible Scanners from China in 2026?
Chinese manufacturers now produce ISO 13485-certified scanners with 20+ micron accuracy, open STL export, and TRIOS workflow compatibility at 40-60% below OEM pricing. Key drivers include:
- Post-pandemic supply chain diversification mandates
- 15% average annual cost reduction in Chinese OEM scanner production (2022-2025)
- 92% of surveyed distributors reporting improved margins with certified Chinese alternatives (2025 DentaTech Report)
Step-by-Step Sourcing Protocol
| Step | Critical Verification Actions | Regulatory Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• Demand original ISO 13485:2016 certificate (not expired) • Validate CE Certificate via EU NANDO database (search NB number) • Require FDA 510(k) or local market registration docs • Confirm scanner-specific certification (not just factory ISO) |
• Certificates issued by non-accredited bodies (e.g., “CE EU 2023”) • Missing MDR 2017/745 compliance date (post-May 2024) • Generic “medical device” certification without scanner classification |
• Non-negotiable: MDR-compliant CE Certificate with NB number • ISO 13485:2016 covering design & production • Traceable UDI in certificate scope |
| 2. Negotiating MOQ & Commercial Terms |
• Standard MOQ: 5-10 units for white-label scanners • Tiered pricing: 15% discount at 20+ units (2026 benchmark) • Demand 18-month warranty with loaner unit provision • Include calibration certificate costs in unit price |
• MOQ below 5 units (indicates substandard production) • Warranty under 12 months • Refusal to provide calibration documentation |
• Max 8-unit MOQ for first order (established partners) • Price parity with EU distributors at 15+ units • Calibration traceable to NIST/PTB standards |
| 3. Shipping & Logistics (2026 Terms) |
• DDP (Delivered Duty Paid) strongly recommended: – Supplier handles China export/customs – All import duties/taxes paid pre-shipment – Door-to-door tracking with Incoterms® 2020 • FOB Shanghai ONLY with bonded warehouse verification |
• Quoted “EXW” terms (buyer assumes China export risk) • No HS code 9018.49.0000 declaration • Refusal to provide DDP duty calculation |
• DDP cost within 8% of FOB + estimated duties • Temperature-controlled packaging (0-40°C) • Real-time GPS tracking mandatory |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier with 19 years of dental equipment export experience (established 2007), Carejoy meets all 2026 sourcing requirements for TRIOS-compatible scanners:
| Verification Point | Carejoy Compliance Status |
|---|---|
| ISO 13485:2016 Certification | ✅ Valid until 03/2027 (Certificate #CN-2023-ISO13485) |
| CE Marking (MDR 2017/745) | ✅ NB 2797 Certified (Scanner Model CJ-IO 5.0) |
| Typical MOQ | 🔹 6 units (first order), 3 units (repeat orders) |
| DDP Logistics | 🔹 Door-to-clinic delivery in 14-18 days (US/EU) |
| Technical Support | 🔹 24/7 English-speaking engineers (SLA: 2-hr response) |
📧 [email protected] (Quote “TRIOS2026-GUIDE”)
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🏭 Facility: 1200m² ISO-certified factory, Baoshan District, Shanghai
🔍 Verification: Request video audit of calibration lab (mandatory for first-time buyers)
2026 Due Diligence Checklist
- Confirm scanner has MDR-compliant CE certificate (not just “CE”) via NANDO
- Require test report showing 20μm accuracy (ISO 12836 standard)
- Verify software compatibility with TRIOS Studio/3Shape Communicate
- Confirm service network in your country (on-site engineers)
- Validate DDP cost breakdown against FOB + 15% logistics buffer
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: 3Shape TRIOS Intraoral Scanner (2026 Model Year)
Target Audience: Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 3Shape TRIOS Intraoral Scanner (2026) support, and is it compatible with international power standards? | The 3Shape TRIOS 2026 series operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it compatible with global electrical systems. Each unit includes region-specific power adapters and complies with IEC 60601-1 safety standards for medical electrical equipment. For distributors, multi-region packaging options are available upon request to support global deployment. |
| 2. What spare parts are recommended to keep in inventory, and how quickly can replacements be sourced? | Key spare components include scanning tips (disposable and reusable), battery modules, handpiece charging docks, and protective sleeves. 3Shape maintains a global network of distribution hubs, ensuring 2–5 business day delivery for standard spare parts in North America, Europe, and Asia-Pacific regions. Authorized distributors receive priority access to inventory and bulk pricing on service kits. |
| 3. What does the installation process involve, and is on-site technician support available? | Installation includes hardware setup, software integration with 3Shape Communicate or your clinic’s existing practice management system, and calibration verification. Remote installation is standard, supported by certified 3Shape technicians via secure connection. On-site installation is available for enterprise clinics or multi-unit rollouts, coordinated through your regional distributor. Training modules and digital onboarding resources are included. |
| 4. What is the warranty coverage for the 3Shape TRIOS scanner, and are extended service plans available? | All 2026 TRIOS scanners include a standard 2-year comprehensive warranty covering parts, labor, and accidental damage (excluding misuse). Extended warranty options are available for up to 5 years, including predictive maintenance, priority technical support, and guaranteed turnaround time for repairs. Distributors may offer bundled service contracts tailored to clinic needs. |
| 5. How are firmware updates and technical support managed post-installation? | Firmware updates are delivered automatically via 3Shape Cloud, ensuring continuous performance improvements and compatibility with new restorative workflows. Technical support is available 24/7 through regional service centers with SLA-backed response times. Registered clinics gain access to the 3Shape Service Portal for remote diagnostics, ticket tracking, and parts ordering. |
Need a Quote for 3Shape Trios Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


