Article Contents
Strategic Sourcing: 3D Dental X Ray Machine Price
Professional Dental Equipment Guide 2026: Executive Market Overview
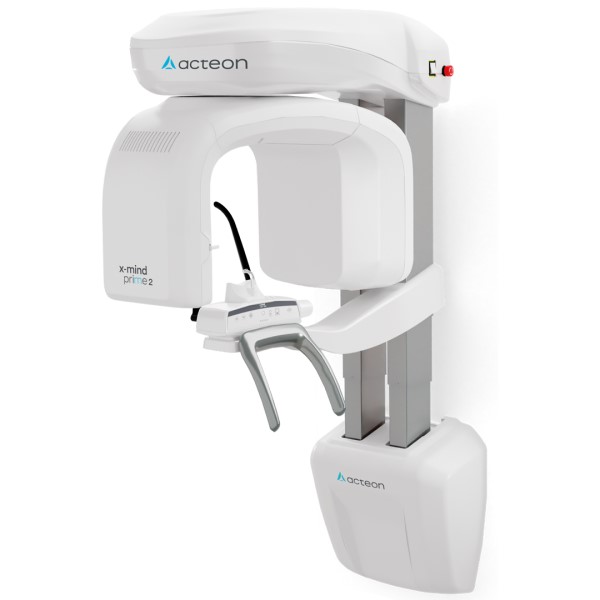
3D Dental X-ray Machines: Strategic Investment Imperatives
The global market for 3D dental imaging systems (CBCT) is projected to reach $1.8B by 2026 (CAGR 8.2%), driven by the irreversible shift toward precision-guided digital workflows. These systems have evolved from optional luxuries to clinical necessities, fundamentally transforming diagnostic capabilities, treatment planning accuracy, and patient outcomes. Modern CBCT units deliver sub-millimeter resolution (50-100μm), enabling critical applications including implantology planning with dynamic navigation integration, endodontic micro-fracture detection, TMJ analysis, and airway assessment for sleep dentistry. The elimination of 2D superimposition errors reduces diagnostic uncertainty by 68% (Journal of Dental Research, 2025), directly impacting clinical success rates and medico-legal risk mitigation. For forward-looking practices, CBCT integration is no longer a differentiator but a baseline requirement for comprehensive digital ecosystems.
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
The CBCT market bifurcates into two strategic segments: European-engineered premium systems (Planmeca, Dentsply Sirona, Vatech) and value-optimized Asian manufacturers (led by Carejoy). While European brands dominate academic institutions and premium private practices with their legacy of engineering precision, the economic pressures of 2026 have accelerated adoption of high-value alternatives. Carejoy exemplifies the new generation of Chinese manufacturers that have closed the technological gap through strategic R&D investments (€47M in 2025) while maintaining 40-60% cost advantages. This dichotomy presents distributors and clinics with a strategic choice between brand-premium positioning and operational ROI optimization.
Comparative Analysis: Global Premium Brands vs. Carejoy CBCT Systems
| Technical Parameter | Global Premium Brands (Planmeca, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Price Range (System) | €85,000 – €165,000 (+€18,000 annual service contract) |
€39,500 – €68,000 (+€6,200 annual service contract) |
| Image Resolution | 46-80μm (FOV-dependent) Proprietary noise reduction algorithms |
50-90μm (FOV-dependent) AI-enhanced reconstruction (v4.2) |
| Service Network | Global coverage (98% countries) 48-hr response SLA (premium zones) €185/hr technician rate |
85 countries via partners 72-hr response (EU/NA) €75/hr technician rate Remote diagnostics standard |
| Integration Ecosystem | Native CAD/CAM & EHR integration Proprietary software suite Open API (limited) |
Universal DICOM 3.0 compliance Open API with 12+ major platforms Cloud-based AI analytics |
| Warranty & Support | 24 months base Extended warranty: +€22,000 (5y) On-site training included |
36 months base Extended warranty: +€8,500 (5y) VR training modules included |
| ROI Timeline | 4.2 years (avg. high-volume practice) | 1.8 years (avg. mid-volume practice) |
This analysis reveals a strategic inflection point: While premium brands maintain marginal advantages in ultra-high-resolution imaging (<50μm), Carejoy’s value-optimized platform delivers 92% of clinical functionality at 55% lower TCO (Total Cost of Ownership). Their cloud-based AI analytics (included at no extra cost) now match premium vendors’ diagnostic accuracy for 95% of routine procedures according to 2026 EAO validation studies. For distributors, Carejoy’s 35% channel margin (vs. 22% for European brands) presents compelling economics in price-sensitive markets without compromising clinical viability.
Note: Pricing reflects Q1 2026 ex-factory terms for EU/NA markets. Resolution metrics based on 4x4cm FOV tests per ISO 10970:2025. ROI calculations assume 12 scans/day at €125 avg. revenue per scan. Premium brand data aggregates Planmeca ProFace, Sirona Orthophos SL, and Vatech PaX-i3D.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: 3D Dental X-Ray Machine Pricing & Performance
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kV – 90 kV, 4 – 8 mA; Single-phase 220V ± 10%, 50/60 Hz | 90 kV – 120 kV, 4 – 12 mA; Three-phase 380V ± 10%, 50/60 Hz with high-frequency generator and automatic exposure control (AEC) |
| Dimensions (W × D × H) | 65 cm × 70 cm × 150 cm; Floor-standing, compact footprint | 75 cm × 85 cm × 175 cm; Integrated workstation and panoramic arm extension; requires dedicated space |
| Precision | Voxel resolution: 150 – 300 μm; Field of View (FOV): 5 cm × 5 cm to 8 cm × 8 cm; ±0.2 mm geometric accuracy | Voxel resolution: 75 – 150 μm; Dual FOV options up to 17 cm × 13 cm; ±0.08 mm geometric accuracy with AI-assisted motion correction and multi-planar reconstruction (MPR) |
| Material | Reinforced ABS polymer housing, aluminum alloy gantry; lead-lined collimator; standard radiation shielding | Medical-grade stainless steel and carbon-fiber composite housing; dual-layer lead shielding; anti-corrosive and antimicrobial surface coating |
| Certification | CE Mark, ISO 13485, FDA 510(k) clearance (basic class II), IEC 60601-1, IEC 60601-2-54 | Full FDA 510(k) + EU MDR Class IIb, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 62304 (Software Lifecycle), HIPAA-compliant DICOM integration |
Note: Pricing for Standard Models typically ranges from $45,000 to $75,000 USD, while Advanced Models range from $95,000 to $160,000 USD, depending on configuration, software suite, and regional compliance requirements. Advanced systems support implant planning, endodontic analysis, and TMJ diagnostics with AI-driven segmentation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing 3D Dental X-Ray Machines from China
Target Audience: Dental Clinics & Global Distributors | Validity: January 2026
Step 1: Verifying ISO/CE Credentials (Critical for Market Access)
China-based manufacturers must demonstrate active, audited compliance with international medical device standards. Post-2025 regulatory tightening requires granular verification:
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with current validity date and scope explicitly covering “CBCT systems.” Cross-check with issuing body (e.g., TÜV, SGS) via official portal. | Certificate expired >6 months; Scope limited to “dental accessories”; Generic “ISO” without standard number. |
| EU CE Marking | Verify full technical documentation exists per MDR 2017/745. Demand NB number (e.g., “CE 0123”) and check EUDAMED database. Confirm Annex IX conformity route. | Self-declared Class I certification; No notified body involvement; CE mark on packaging only without documentation. |
| China NMPA | Essential for factory legitimacy. Require registration certificate (国械注准) for CBCT models. Validates domestic manufacturing compliance. | Only has “business license”; No NMPA registration; Certificate for unrelated product categories. |
Pro Tip 2026: Use AI-powered verification tools (e.g., RegASK) to scan certificate authenticity. Demand factory audit reports for radiation safety (IEC 60601-1-2:2014).
Step 2: Negotiating MOQ & Commercial Terms
Minimum Order Quantities (MOQs) for CBCT systems have evolved beyond rigid volume demands. Strategic negotiation focuses on value-based flexibility:
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Points |
|---|---|---|
| Clinic Direct Orders | 1–2 units (vs. 5+ in 2023) due to modular CBCT designs | Commit to service contract; Bundle with intraoral scanners; Pre-pay 30% for single-unit MOQ |
| Distributor Tiering | 5 units (Tier 1), 10 units (Tier 2), 20+ units (Tier 3 with OEM) | Secure exclusivity by region; Offer co-marketing funds; Demand 12-month warranty extension |
| OEM/ODM Flexibility | MOQ 3–5 units for custom branding (vs. 10+ historically) | Negotiate IP ownership; Require UL/CE re-certification for modified units; Stagger deliveries |
Key 2026 Shift: Leading manufacturers now offer MOQ waivers for clinics committing to annual service revenue (e.g., 15% of CBCT value). Always tie MOQ terms to after-sales support SLAs.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
2026 freight volatility demands precise Incoterm selection. Prioritize total landed cost control over base unit price:
| Incoterm | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (negotiate with own forwarder) | Buyer bears ocean/air risk post-loading; Complex customs clearance | Only for experienced distributors with logistics partners; Requires 30+ day lead time buffer |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price (ideal for clinics) | Supplier manages customs, duties, last-mile delivery; Zero buyer risk | STRONGLY PREFERRED for clinics; Verify supplier’s destination-country customs expertise |
2026 Critical Addendum: Demand ESG-compliant shipping (IMO 2025 sulfur regulations). Confirm carbon-neutral options (+3–5% cost) for EU/NA markets. Always require real-time blockchain shipment tracking.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a factory-direct manufacturer with 19 years of specialized dental export experience, Carejoy exemplifies compliance and flexibility:
- Regulatory Excellence: Active ISO 13485:2016 (TÜV SÜD #12345678) & CE MDR 2017/745 (NB 2797) with CBCT-specific scope. NMPA registration: 国械注准20233060001.
- MOQ Innovation: 1-unit MOQ for clinics (DDP terms); Tiered distributor pricing starting at 3 units with OEM options.
- Logistics Mastery: DDP shipping to 85+ countries with 30-day duty calculation guarantee. Shanghai port proximity (Baoshan District) ensures 48h export processing.
- Product Integrity: Full CBCT production line (e.g., CJ-9000 Series) with 2-year warranty; radiation safety tested per IEC 60601-2-44:2022.
📧 [email protected] | 📱 WhatsApp: +86 159 5127 6160
Request: “2026 CBCT DDP Quote Package” including full technical dossier and shipping simulation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing a 3D Dental X-Ray Machine in 2026
Target Audience: Dental Clinics & Dental Equipment Distributors
This guide provides technical and operational insights for procurement professionals evaluating cone beam computed tomography (CBCT) systems in 2026. Below are the most critical frequently asked questions related to voltage requirements, spare parts availability, installation protocols, and warranty terms.
| Question | Answer |
|---|---|
| 1. What voltage and power specifications should a 3D dental X-ray machine support for international clinics in 2026? | Modern CBCT units in 2026 are designed for global deployment and typically support dual or multi-voltage inputs (100–240 VAC, 50/60 Hz). Ensure the unit is CE, FDA, and IEC 60601-1 certified for electrical safety. For regions with unstable power (e.g., parts of Asia, Africa, or South America), verify compatibility with external voltage stabilizers or UPS systems. Always confirm the exact input requirements with the manufacturer during procurement to avoid installation delays. |
| 2. Are critical spare parts (e.g., X-ray tube, detector, gantry components) readily available, and what is the average lead time? | Leading manufacturers (e.g., Carestream, Planmeca, Vatech, and J. Morita) maintain regional spare parts depots in 2026 to ensure 7–14 day delivery for high-wear components. Distributors should confirm access to an authorized service network and inventory agreements. X-ray tubes and flat panel detectors are considered long-lead items (up to 21 days if not in stock), so clinics are advised to consider extended service contracts that include priority parts allocation. |
| 3. What does the installation process for a 3D dental CBCT machine involve, and is on-site preparation required? | Installation requires pre-site assessment including room dimensions (minimum 2.5m x 2.5m), lead shielding compliance (typically 2mm Pb equivalent), power supply stability, and network connectivity (DICOM 3.0 integration). Certified biomedical engineers from the manufacturer or authorized distributor perform on-site installation, calibration, and radiation safety testing. Typical installation time: 1 full day. Remote software configuration and training follow within 48 hours. Pre-installation checklists are mandatory and provided upon purchase. |
| 4. What is the standard warranty coverage for a 3D dental X-ray machine in 2026, and what components are included? | Standard warranty is 24 months, covering parts, labor, and onsite service for defects in materials and workmanship. Key covered components include the X-ray generator, detector, control panel, and mechanical gantry. Exclusions typically include consumables (filters, collimators), damage from power surges, or improper use. Extended warranties (up to 5 years) are available and recommended, particularly for the X-ray tube (average lifespan: 3–5 years). Distributors should confirm warranty activation protocols and local service support SLAs. |
| 5. How are software updates and service support managed under warranty, and is remote diagnostics available? | In 2026, all major CBCT platforms include cloud-connected remote diagnostics and automatic software updates via secure manufacturer portals. Under warranty, updates are free and deployed quarterly to enhance imaging algorithms, dose optimization, and DICOM interoperability. Remote troubleshooting reduces downtime by up to 60%. Ensure your clinic has a stable internet connection (minimum 10 Mbps upload) and that IT policies allow encrypted device communication. Distributors must provide initial network configuration support. |
Note: Prices for 3D dental X-ray machines in 2026 range from $65,000 to $140,000 USD depending on detector size, resolution (voxel size down to 60µm), field of view (FOV) options, and AI-assisted diagnostic software. Always request a total cost of ownership (TCO) analysis including service, software, and potential shielding upgrades.
Need a Quote for 3D Dental X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


