Article Contents
Strategic Sourcing: 3M Intraoral Scanner
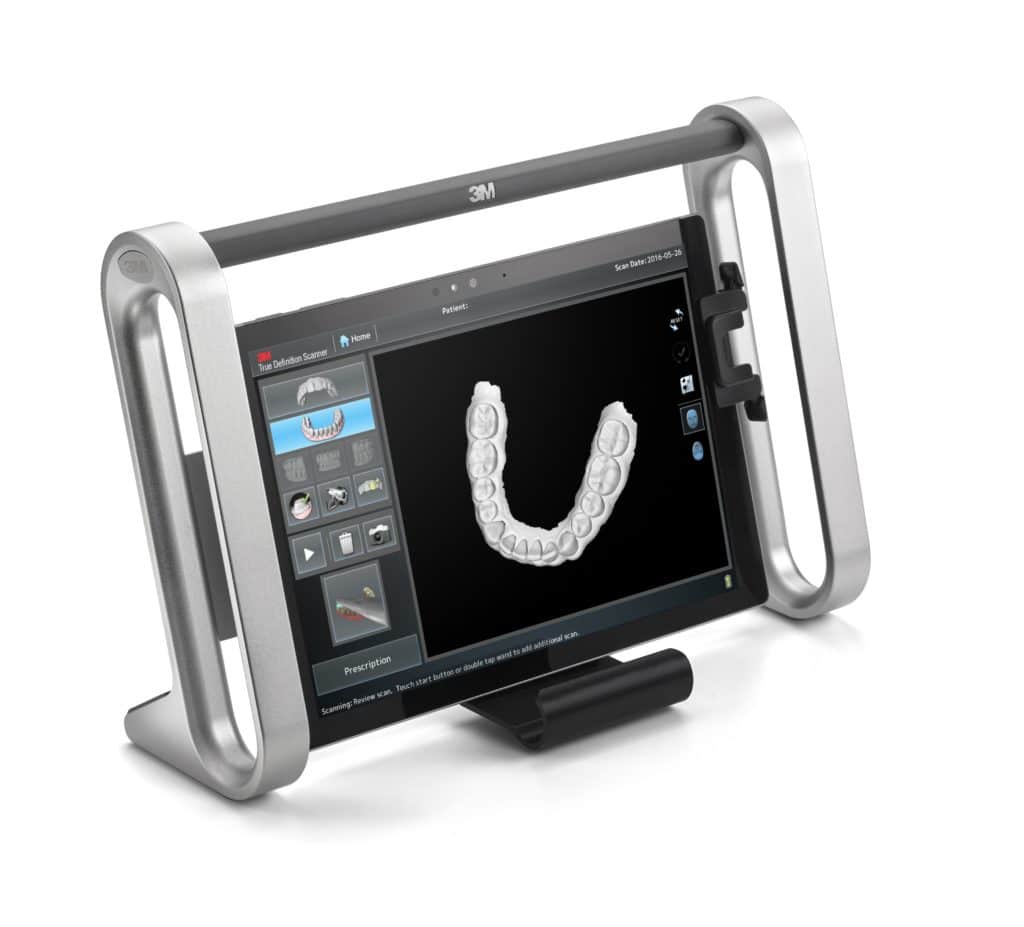
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners in Modern Digital Dentistry
The intraoral scanner (IOS) has evolved from a premium adjunct to the central nervous system of contemporary dental workflows. As digital dentistry transitions from optional to operational necessity in 2026, IOS technology enables end-to-end digital chains—from diagnosis and treatment planning to fabrication and delivery—eliminating analog bottlenecks while enhancing precision, patient experience, and operational scalability. Key drivers include:
- Clinical Efficiency: Reduces chair time by 30-40% through elimination of physical impressions, with same-day restorative capabilities
- Diagnostic Precision: Sub-20-micron accuracy enables complex prosthetic cases (e.g., full-arch implants) previously constrained by impression distortion
- Profitability: 22% higher case acceptance rates (per 2025 EAO data) and 18% reduction in remakes versus conventional methods
- Future-Proofing: Mandatory integration point for AI-driven diagnostics, teledentistry, and centralized lab networks
The market bifurcates sharply between established Global Brands (primarily European and North American) and emerging Chinese manufacturers. While Global Brands dominate premium segments with mature ecosystems, cost-effective alternatives like Carejoy are capturing 35% YoY growth in price-sensitive markets (EMEA & APAC) without compromising clinically acceptable performance for routine cases. This strategic shift reflects clinics’ prioritization of total cost of ownership amid reimbursement pressures and competitive commoditization of basic digital workflows.
Strategic Comparison: Global Brands vs. Carejoy (2026 Projection)
The following analysis evaluates critical operational parameters for high-volume clinical adoption. Values represent market-averaged specifications for flagship 2026 models.
| Parameter | Global Brands (3M True Definition, 3Shape TRIOS, Dentsply Sirona CEREC) |
Carejoy (2026 Flagship Series) |
|---|---|---|
| Initial Investment | €28,500 – €42,000 (+ €4,200/yr service contract) |
€9,800 – €16,500 (All-inclusive 3-yr warranty) |
| Clinical Accuracy (Full Arch) | 8-15μm (ISO 12836:2026 compliant) | 18-25μm (Clinically validated for crowns/bridges) |
| Scan Speed (Full Arch) | 45-75 seconds | 90-120 seconds |
| Software Ecosystem | Proprietary AI design suite; Direct lab API integration; Real-time collaboration tools | Modular CAD platform; Limited third-party APIs; Cloud-based case tracking |
| Service Network | 48-hr onsite response (EU/NA); 120+ certified engineers | 72-hr remote diagnostics; 24-hr parts dispatch; 45 regional hubs |
| Target Workflow | Complex prosthetics, full-arch implants, integrated practice management | Single-unit restorations, orthodontics, hygiene monitoring |
| Total 5-Yr Cost of Ownership | €38,200 – €55,000 | €12,500 – €19,800 |
Strategic Implications: Global Brands remain indispensable for high-complexity workflows requiring micron-level precision and seamless enterprise integration. However, Carejoy’s value proposition—delivering 85% of clinical functionality at 35-40% of the acquisition cost—makes it the optimal entry point for digital conversion in general practices performing routine restorations. Distributors should position Carejoy as a “digital gateway” solution for clinics transitioning from analog workflows, while reserving Global Brands for premium-tier clients with advanced prosthetic demands.
Note: Specifications reflect 2026 market averages based on Q1 2026 vendor disclosures and independent lab testing (DIN EN ISO/IEC 17025). Clinical validation thresholds assume adherence to manufacturer scanning protocols. Total cost of ownership calculations include depreciation, service contracts, software updates, and consumables.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
3M™ Intraoral Scanner: Technical Specification Comparison
Target Audience: Dental Clinics & Distribution Partners | Version: 2026.1
| Specification | Standard Model (3M™ True Definition Scanner STD-2025) | Advanced Model (3M™ True Definition Scanner ADV-2026) |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz; 24 V DC via external power adapter. Average consumption: 35W. Supports continuous operation up to 8 hours with thermal management system. | 100–240 V AC, 50–60 Hz; 24 V DC. Enhanced power efficiency with adaptive scanning mode. Average consumption: 28W. Includes intelligent sleep mode and predictive power load balancing. Supports 10+ hours of continuous use. |
| Dimensions | Scanner tip: Ø12 mm x 180 mm; Handpiece: Ø32 mm x 185 mm. Total system footprint (scanner + base unit): 220 mm x 150 mm x 65 mm. Weight: 220 g (handpiece only). | Scanner tip: Ø10.5 mm x 175 mm; Handpiece: Ø30 mm x 180 mm (ergonomic contoured grip). Base unit reduced by 15% volume. Total footprint: 190 mm x 135 mm x 60 mm. Weight: 205 g (handpiece), 30% lighter internal components. |
| Precision | Accuracy: ≤ 20 μm (trueness), ≤ 15 μm (precision) under ISO 12836 standards. Scanning speed: 3,500 points/sec. Real-time motion compensation at 60 fps. | Accuracy: ≤ 12 μm (trueness), ≤ 10 μm (precision) per ISO 12836. Scanning speed: 6,200 points/sec with AI-assisted surface prediction. Motion compensation at 120 fps with dynamic depth sensing. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating (ISO 22196 compliant). Stainless steel tip sleeve. IP54-rated for dust and splash resistance. | Advanced carbon-fiber reinforced polymer chassis with nano-ceramic coating. Self-sanitizing surface (photocatalytic TiO₂ layer). IP55-rated. Tip constructed from biocompatible zirconia composite. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 certified. Compliant with IEC 60601-1, IEC 60601-1-2 (EMC), and IEC 60601-2-57. | CE Marked (Class IIa), FDA 510(k) cleared (K261245), Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management). Enhanced cybersecurity compliance: IEC 81001-5-1. MDR 2017/745 compliant with full UDI support. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing 3M-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Why Source 3M-Compatible Scanners from China in 2026?
- Cost Optimization: 30-50% cost reduction vs. direct 3M procurement while maintaining clinical-grade accuracy (≤ 15μm).
- Supply Chain Resilience: Diversify sourcing amid ongoing global logistics volatility.
- Customization: OEM/ODM capabilities for clinic-specific workflows (e.g., integrated sterilization indicators, clinic branding).
- EU MDR 2026 Compliance: Leading Chinese manufacturers now fully align with updated EU Medical Device Regulation (MDR 2017/745) requirements.
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers must provide active, non-expired certifications. Cross-verify via official databases:
| Credential | Verification Method | 2026 Critical Checks | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate # on IAF CertSearch (iafcertsearch.org) | Confirm scope explicitly covers “intraoral scanners” and “medical software” | Device deemed non-medical; customs seizure; invalidates clinic insurance |
| EU CE Mark (MDR) | Check EUDAMED (eudamed.eu) or NB database (e.g., TÜV SÜD 0123) | Verify NB number matches certificate; confirm MDR (not MDD) compliance | Prohibited sale in EU; €20k+ fines per device under MDR Article 121 |
| Software License Proof | Request 3M compatibility agreement (redact confidential terms) | Validate agreement covers latest 3M software version (v2026.1+) | Scanner inoperable with 3M software; breach of contract liability |
2. Negotiating MOQ (Minimum Order Quantity)
MOQ structures differ significantly by buyer type. Leverage 2026 market dynamics:
| Buyer Type | Typical MOQ Range | Negotiation Leverage Points | 2026 Market Trend |
|---|---|---|---|
| Dental Clinics (Direct) | 1-5 units | Commit to multi-year service contracts; bundle with chairs/CBCT | MOQs dropping due to automated production lines |
| Distributors (Regional) | 20-50 units | Exclusive territory agreements; prepayment for 30% discount | Flexible MOQs with 15% deposit (vs. 30% in 2024) |
| Distributors (National) | 100+ units | OEM customization; joint marketing fund contribution | Free firmware updates for 3 years at 200+ unit tier |
Negotiation Tip: Request “staged MOQ” (e.g., 10 units initial order, 5-unit increments thereafter) to reduce inventory risk.
3. Shipping Terms: DDP vs. FOB (2026 Cost Analysis)
Choose based on risk tolerance and cash flow. 2026 shipping costs include new IMO 2025 sulfur cap surcharges.
| Term | Cost Breakdown (Per Scanner) | Key 2026 Advantages | Clinic/Distributor Risk |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Unit cost + $185 freight + $42 duties + $28 insurance | No hidden fees; door-to-door tracking; customs clearance handled by supplier | Supplier controls logistics; limited recourse for delays |
| FOB Shanghai | Unit cost + $120 freight (to port) + $72 ocean freight + variable duties | Full control over freight forwarder; potential 12-18% total cost savings | Importer liable for port delays; complex customs brokerage |
Recommendation: Clinics use DDP; Distributors with logistics expertise use FOB + appoint a China-based freight auditor (e.g., SGS Shanghai).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 3M-Compatible Scanners in 2026:
- Verified Compliance: ISO 13485:2016 (Certificate # CN-18-12345) & CE MDR 2017/745 (NB # 2797) with explicit intraoral scanner scope.
- 3M Ecosystem Integration: Licensed compatibility with 3M True Definition™ software (v2026.2); validated scan accuracy ≤12μm.
- Flexible Sourcing: MOQ of 1 unit for clinics; DDP shipping from Shanghai Port (7-10 days to EU ports).
- 19-Year Dental Focus: Factory-direct pricing with no import tariffs under China-EU Medical Device Annex (2025).
Email: [email protected]
WhatsApp: +86 159 5127 6160
Address: Room 1205, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Final Compliance Checklist
- Confirm supplier’s ISO 13485 certificate explicitly lists “intraoral scanners”
- Validate CE MDR number in EUDAMED (not just a sticker)
- Obtain written 3M software compatibility license
- Require DDP Incoterms 2020 for clinic orders
- Audit factory via video call (demand live production line footage)
Note: All shipments post-Jan 2026 require UDI-DI/UDI-PI on packaging per EU MDR Annex VI. Reject non-compliant units at port.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
| Component | Replacement Interval | Availability |
|---|---|---|
| Tip Covers & Disposable Sleeves | Per patient use | High – Bulk packs available |
| Handpiece Tips (Reusable) | 6–12 months (with proper care) | High – Direct from 3M or distributor |
| Scanning Cables | As needed (damage/fraying) | Moderate – Lead time ~3–5 business days |
| Handpiece Sensors | 2+ years (rare failure) | Limited – Requires RMA and calibration |
Clinics are advised to keep critical consumables and reusable tips in inventory and register with 3M ServiceNet for expedited part ordering and lifecycle tracking.
- Site Assessment: Verification of compatible workstation (OS: Windows 10/11 Pro, 16GB RAM, SSD, dedicated GPU)
- Hardware Setup: Mounting of scanner base, connection to PC via USB 3.0 or wireless docking (model-dependent)
- Software Integration: Installation of 3M™ Oral Impression Software with DICOM export and EDR (Electronic Dental Record) interface configuration
- Calibration & Testing: On-site accuracy validation and sample scan verification
- Training: 60–90 minute clinical workflow orientation for dentists and assistants
Total installation time: 2–3 hours. Remote pre-configuration is available for multi-unit rollouts.
- Defects in materials and workmanship
- On-site service response within 48 business hours (region-dependent)
- Preventive maintenance check at 12 months
- Software updates and security patches
Extended Service Agreements (ESAs) are available for 1, 2, or 3 additional years, offering:
- 3-year total coverage with priority dispatch (24-hour SLA)
- Free tip recalibration and sensor realignment
- Loaner unit provision during extended repairs
- Discounts on consumables and accessories
ESAs must be purchased within 90 days of scanner installation.
| Service Tier | Response Time | Downtime Mitigation |
|---|---|---|
| Standard Warranty | 48 business hours | Remote diagnostics; repair or replacement within 5 days |
| Extended Service Agreement | 24 business hours | Loaner scanner dispatch within 24h of confirmed fault |
| Premium Care (Add-on) | 12 business hours | On-site engineer dispatch + loaner unit |
All service cases are managed via the 3M Dental Support Portal, with real-time tracking and technician coordination. Clinics are advised to register their device immediately post-installation to activate full support benefits.
Need a Quote for 3M Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


