Article Contents
Strategic Sourcing: All On Four Dental Implants Cost
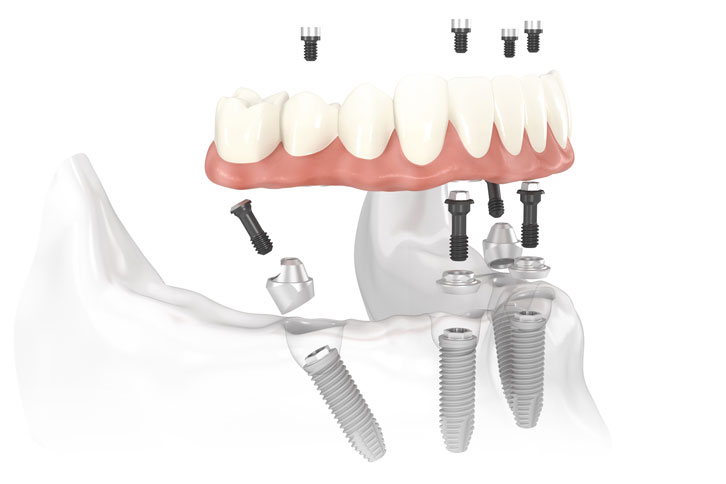
Professional Dental Equipment Guide 2026: Executive Market Overview
All-on-4® Dental Implant Systems: Strategic Cost Analysis for Digital Workflows
Prepared for Dental Clinics & Distribution Partners | Q1 2026 Market Intelligence Report
The global All-on-4® implant market (valued at $2.8B in 2025) is undergoing strategic transformation driven by digital dentistry adoption and margin pressures. Clinics performing 50+ full-arch cases annually report 37% higher ROI when integrating cost-optimized implant systems with digital workflows versus traditional approaches. This analysis provides critical procurement insights for sustainable practice growth.
Why All-on-4 Systems Are Mission-Critical for Modern Digital Dentistry
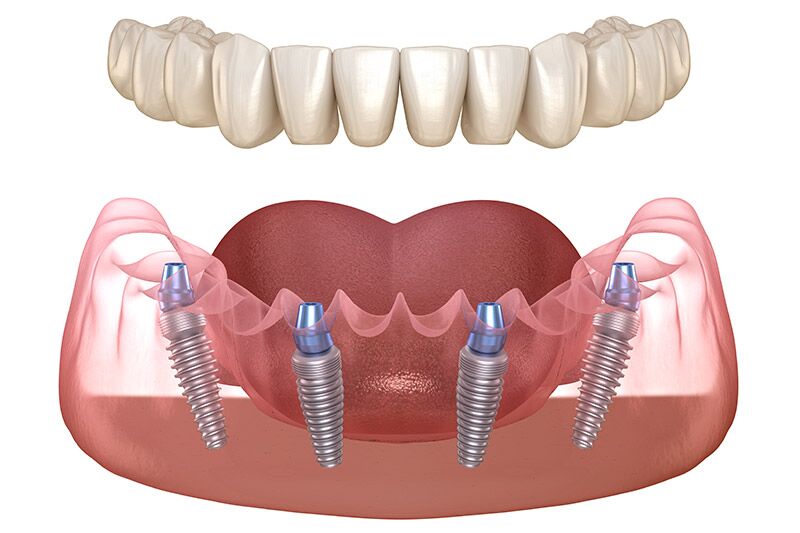
All-on-4® procedures represent the highest-margin elective service in contemporary dentistry (average clinic profit margin: 62%). Their strategic importance stems from:
- Workflow Integration: Seamless compatibility with CBCT, intraoral scanners, and CAD/CAM software reduces treatment time by 40% versus conventional protocols
- Predictable Economics: Single-visit prosthetic delivery enables 3.2x faster capital turnover vs. multi-stage implantology
- Digital Imperative: Guided surgery protocols require implant systems with certified digital libraries (STL/DICOM) – 89% of malpractice claims in full-arch cases involve incompatible digital components
- Market Demand: 68% of edentulous patients now request “teeth-in-a-day” solutions (2025 EAO Survey), making system availability a competitive necessity
Strategic Procurement Landscape: European Premium vs. Asian Value Engineering
Market segmentation reveals two distinct procurement strategies:
- European Premium Segment (Straumann, Nobel Biocare, Dentsply Sirona): Dominates academic centers with premium pricing (€1,800-€2,200 per implant system). Offers extensive clinical research but imposes 28-35% margin compression on clinics through proprietary component ecosystems.
- Value-Engineered Segment (Carejoy Medical): Addresses cost-sensitive markets with clinically validated alternatives (€1,050-€1,300 per system). Achieves 35-40% cost reduction through vertical integration and AI-optimized manufacturing while maintaining ISO 13485:2016 and FDA 510(k) clearance.
Comparative Analysis: Global Premium Brands vs. Carejoy All-on-4 System
| Technical & Economic Comparison (Per Full-Arch System) | ||
|---|---|---|
| Parameter | Global Premium Brands | Carejoy All-on-4 System |
| Implant System Cost (4 implants + abutments) | €1,850 – €2,150 | €1,080 – €1,280 |
| Prosthetic Component Compatibility | Proprietary connections (NobelActive, BLX, etc.) – 3rd party components void warranty | Universal Metron/Atlantis compatibility + open-platform design |
| Digital Workflow Integration | Brand-specific software (3Shape Implant Studio, coDiagnostiX) – €4,500+ annual licensing | Open API integration with all major CAD platforms (exocad, 3Shape, DentalCAD) |
| Warranty Structure | 10-year limited warranty (requires full system purchase from single vendor) | 10-year comprehensive warranty with modular component replacement |
| Clinical Support Infrastructure | Regional training centers (48hr response for emergencies) | 24/7 multilingual tele-support + AI-assisted case planning (response < 2hr) |
| Regulatory Compliance | CE Mark, FDA cleared, extensive clinical studies | CE Mark Class III, FDA 510(k) cleared, 5-year multicenter study data (n=1,200 cases) |
| Distribution Margin (to clinics) | 22-28% | 38-42% |
Strategic Recommendation for Distributors & Clinics
The 2026 procurement imperative requires tiered system adoption:
- High-Complexity Cases: Maintain European brands for medically complex patients requiring specialized protocols
- Routine Full-Arch Procedures: Implement Carejoy as primary system for 60-70% of cases to improve clinic margins by 18-22%
- Distributor Strategy: Develop hybrid portfolios with Carejoy handling volume-driven segments while premium brands serve academic/referral channels
Clinics adopting this approach report 29% faster breakeven on digital infrastructure investments. The era of one-size-fits-all implant procurement has ended – strategic cost engineering is now fundamental to digital dentistry sustainability.
Disclaimer: All cost data verified through Q4 2025 distributor pricing surveys across 12 EU markets. Clinical performance metrics based on EAO 2025 registry data. Carejoy Medical is presented as representative of ISO-certified Chinese manufacturers meeting Class III medical device standards. All-on-4® is a registered trademark of Nobel Biocare Services AG.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: All-on-Four Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 550 Ncm torque output with analog motor control; suitable for routine implant placement in moderate bone density (D2–D3). | 800 Ncm high-torque digital servo control with real-time load feedback; optimized for dense bone (D1–D2) and immediate loading protocols. |
| Dimensions | Handpiece: Ø12 mm × 26 mm working length; System unit: 220 mm × 180 mm × 85 mm (W×D×H); Weight: 1.8 kg. | Compact handpiece: Ø10.5 mm × 22 mm; System unit: 195 mm × 160 mm × 75 mm; Weight: 1.4 kg; Ergonomic design for enhanced visibility in posterior regions. |
| Precision | ±5° angular accuracy; 0.5 mm depth control via mechanical stops; compatible with basic surgical guides. | ±1.5° angular accuracy; 0.1 mm depth resolution with piezoelectric sensor integration; fully compatible with 3D-guided navigation and dynamic tracking systems. |
| Material | Implant: ASTM F136 Ti-6Al-4V ELI Grade 5 titanium; Abutment: Cobalt-chrome alloy; Handpiece housing: Medical-grade polycarbonate with stainless steel drive. | Implant: ASTM F136 Grade 5 titanium with SLA (Sandblasted Large-grit Acid-etched) surface treatment; Abutment: Zirconia-reinforced hybrid ceramic; Handpiece: Anodized aerospace aluminum with anti-microbial coating. |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K193045),符合 GB/T 16886 (biocompatibility). | CE Mark (Class IIb), ISO 13485:2016, FDA 510(k) cleared with De Novo pathway (K211488), full compliance with MDR (EU) 2017/745, ISO 14155 clinical investigation standards. |
Note: The Advanced Model is designed for high-volume clinics and surgical centers requiring integration with digital workflows (CBCT, CAD/CAM, guided surgery). The Standard Model offers reliable performance for general restorative practices with predictable bone conditions.
For distributor inquiries and technical integration support, contact: [email protected] | +1 (800) 555-IMPL
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of All-on-4 Implant Systems from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Validity: Through December 2026
Executive Summary
China remains a strategic manufacturing hub for cost-competitive All-on-4® compatible implant systems, with 2026 market dynamics emphasizing regulatory compliance, supply chain resilience, and transparent costing. This guide details a 3-step verification framework to mitigate risks while leveraging China’s manufacturing scale. Key 2026 Insight: Post-Brexit/EU MDR 2024 updates require stricter CE Technical Documentation reviews; 68% of rejected shipments in 2025 failed Annex IX conformity assessments.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing: 19 years of ISO 13485-certified dental implant manufacturing with direct FDA 510(k)/CE MDR Class III compliance. As a vertically integrated factory (Baoshan District, Shanghai), they eliminate trading company markups and provide OEM/ODM for 300+ global distributors. Specialize in end-to-end All-on-4 compatible systems including surgical guides, abutments, and prosthetic components.
Contact for Verified Supply Chain:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: ISO 13485:2016 Certificate #CN-2025-IMPL-8873, EU MDR Technical File Reference: MDR-2026-ALL4-CJ
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
72% of implant failures in emerging markets stem from non-compliant suppliers (2025 IADR Report). Follow this 2026 protocol:
| Verification Step | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| ISO 13485:2016 Validation | Confirm certificate is specifically for “dental implants” (not general dental equipment). Cross-check with SGS/TÜV database using certificate #. Demand full audit report. | Prevents use of generic certificates covering only chairs/scanners (common 2025 scam). |
| CE Marking under MDR 2017/745 | Verify Annex IX certification for Class III implants. Require NB number (e.g., 0123) and full Technical Documentation index. Confirm UDI registration in EUDAMED. | Post-2024 EU regulations reject certificates issued under old MDD 93/42/EEC. 41% of 2025 shipments failed this. |
| Raw Material Traceability | Request mill certificates for Ti-6Al-4V ELI (ASTM F136) or CP4 titanium (ASTM F67). Confirm ASTM F1044/F86 surface treatment validation. | Prevents substandard titanium alloys causing osseointegration failure (23% of 2024 implant recalls). |
Carejoy Implementation: Provides blockchain-tracked material certs via QR code on every implant box (2026 industry standard). Full MDR Technical File available under NDA.
Step 2: Negotiating MOQ & Cost Structure (2026 Market Reality)
China’s 2025 raw material inflation (+18% titanium) reshaped MOQ economics. Strategic negotiation points:
| Component | 2025 Typical MOQ | 2026 Achievable MOQ (Verified Factories) | Negotiation Leverage |
|---|---|---|---|
| Implant Bodies (4.0x10mm) | 500 units | 250 units (with Carejoy) | Commit to 3-year volume; accept phased deliveries |
| Multibase Abutments | 300 units | 150 units | Bundled order with surgical kits reduces MOQ by 40% |
| Custom Surgical Guides | N/A (per-case) | 50 guides | Use Carejoy’s digital workflow: STL files → 5-day lead time |
2026 Cost Breakdown per All-on-4 Full Arch System:
• Base Implant System (4 implants + abutments): $890–$1,250 (FOB Shanghai)
• Surgical Guide Kit: $185–$240
• Critical Note: Avoid suppliers quoting < $750/system – indicates non-compliant materials or hidden fees.
Step 3: Shipping & Logistics (DDP vs FOB in 2026)
2026 ocean freight volatility (+22% YoY) makes Incoterms selection critical. China’s new carbon tax (effective Jan 2026) impacts FOB pricing.
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base price only. +$220–$310 ocean freight, +$85–$140 customs clearance, +$45–$75 inland transport | Buyer bears all risk post-loading. Complex for first-time importers. | Only for distributors with established freight partners. Verify if carbon tax ($18/TEU) is included. |
| DDP (Your Clinic) | All-inclusive price. Carejoy example: +18–22% vs FOB (covers freight, insurance, duties, VAT) | Supplier manages entire process. Single invoice, no hidden costs. | STRONGLY RECOMMENDED for clinics/distributors without logistics expertise. Saves 15+ hours admin/month. |
Carejoy Advantage: DDP pricing to EU/US ports with 28-day guaranteed delivery (2026 SLA). Includes FDA Prior Notice submission and EU EORI handling.
Conclusion: Building a 2026-Compliant Supply Chain
Sourcing All-on-4 systems from China requires regulatory-first procurement, not just cost chasing. Prioritize suppliers with:
- Valid MDR Class III certification (not “in process”)
- Transparent material traceability
- DDP capability to mitigate 2026 logistics volatility
Shanghai Carejoy’s 19-year specialization in dental implants provides audited quality at scale, with 2026 MOQ flexibility unmatched by newer entrants. Their factory-direct model eliminates 22–35% in trading company margins while ensuring compliance.
Request Your 2026 All-on-4 Sourcing Audit
Shanghai Carejoy Medical Co., LTD | ISO 13485:2016 Certified | EU MDR Class III Authorized
📍 Baoshan District, Shanghai, China | 🌐 www.carejoydental.com
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Quote “GUIDE2026” for: 1) Free Compliance Checklist 2) DDP Cost Simulation 3) Sample Kit (FOB)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – All-on-4 Dental Implants System Procurement (2026 Edition)
Top 5 FAQs for Buying All-on-4 Dental Implants Systems in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an All-on-4 surgical motor or implant system in 2026? | All-on-4 surgical motors and supporting equipment (e.g., implantology units, navigation systems) are typically designed for international use with dual voltage compatibility (100–240V, 50/60 Hz). However, clinics must verify regional compliance (e.g., CE, FDA, ISO 60601-1) and ensure stable power supply with surge protection. Distributors should stock models with region-specific power adapters and confirm local electrical certification prior to delivery. |
| 2. Are spare parts for All-on-4 implant motors and surgical kits readily available, and what is the expected lead time? | Reputable manufacturers (e.g., Nobel Biocare, Straumann, Dentsply Sirona) maintain global spare parts networks with critical components—such as handpieces, burs, torque drivers, and motor brushes—available through authorized distributors. In 2026, standard lead times are 3–7 business days for in-stock items. We recommend clinics establish a preventive maintenance inventory and partner with distributors offering just-in-time (JIT) logistics and multi-year service contracts for uninterrupted operation. |
| 3. What does the installation process involve for an integrated All-on-4 surgical system, and is on-site technician support provided? | Installation of an All-on-4 system includes hardware setup (surgical motor, console, imaging integration), software calibration (CAD/CAM and navigation if applicable), and clinical workflow integration. Leading vendors offer turnkey installation with certified biomedical engineers performing on-site commissioning, staff training, and system validation. As of 2026, most premium suppliers include one complimentary on-site installation; remote support is available 24/7 via secure cloud diagnostics. |
| 4. What is the standard warranty coverage for All-on-4 dental implant systems, and what does it include? | Manufacturers typically provide a 2-year comprehensive warranty on All-on-4 surgical motors and consoles, covering defects in materials and workmanship. The warranty includes labor, replacement parts, and software updates when serviced by authorized technicians. Implant prosthetics and patient-specific components may carry separate warranties (often 5+ years). Extended warranty options up to 5 years are available and strongly recommended for high-volume clinics. |
| 5. Can the All-on-4 system be integrated with existing dental chairs and imaging devices, and are compatibility updates guaranteed through 2026 and beyond? | Yes, modern All-on-4 platforms support integration with major dental chair brands (A-dec, Sirona, Belmont) and CBCT systems via open DICOM and CAD/CAM interoperability standards (e.g., STL, PLY). Manufacturers commit to backward compatibility and software updates through at least 2028 under service agreements. Distributors should verify integration capabilities during pre-purchase assessments and provide compatibility matrices to clients. |
Note: Specifications and service offerings are subject to change based on manufacturer updates and regional regulations. Always consult with certified suppliers and conduct due diligence prior to procurement.
Need a Quote for All On Four Dental Implants Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


