Article Contents
Strategic Sourcing: Average Cost Of Dental Implants Per Tooth
Professional Dental Equipment Guide 2026
Executive Market Overview: Average Cost of Dental Implants Per Tooth
The global dental implant market is projected to reach $12.3B by 2026 (CAGR 9.8%), with significant cost stratification emerging between premium European manufacturers and value-driven Asian suppliers. Current average costs per implant unit range from $1,800–$3,200 for established European brands versus $650–$1,100 for certified Chinese manufacturers like Carejoy. This 40-65% cost differential is reshaping procurement strategies as clinics balance clinical outcomes with economic viability, particularly in price-sensitive markets and high-volume practices.
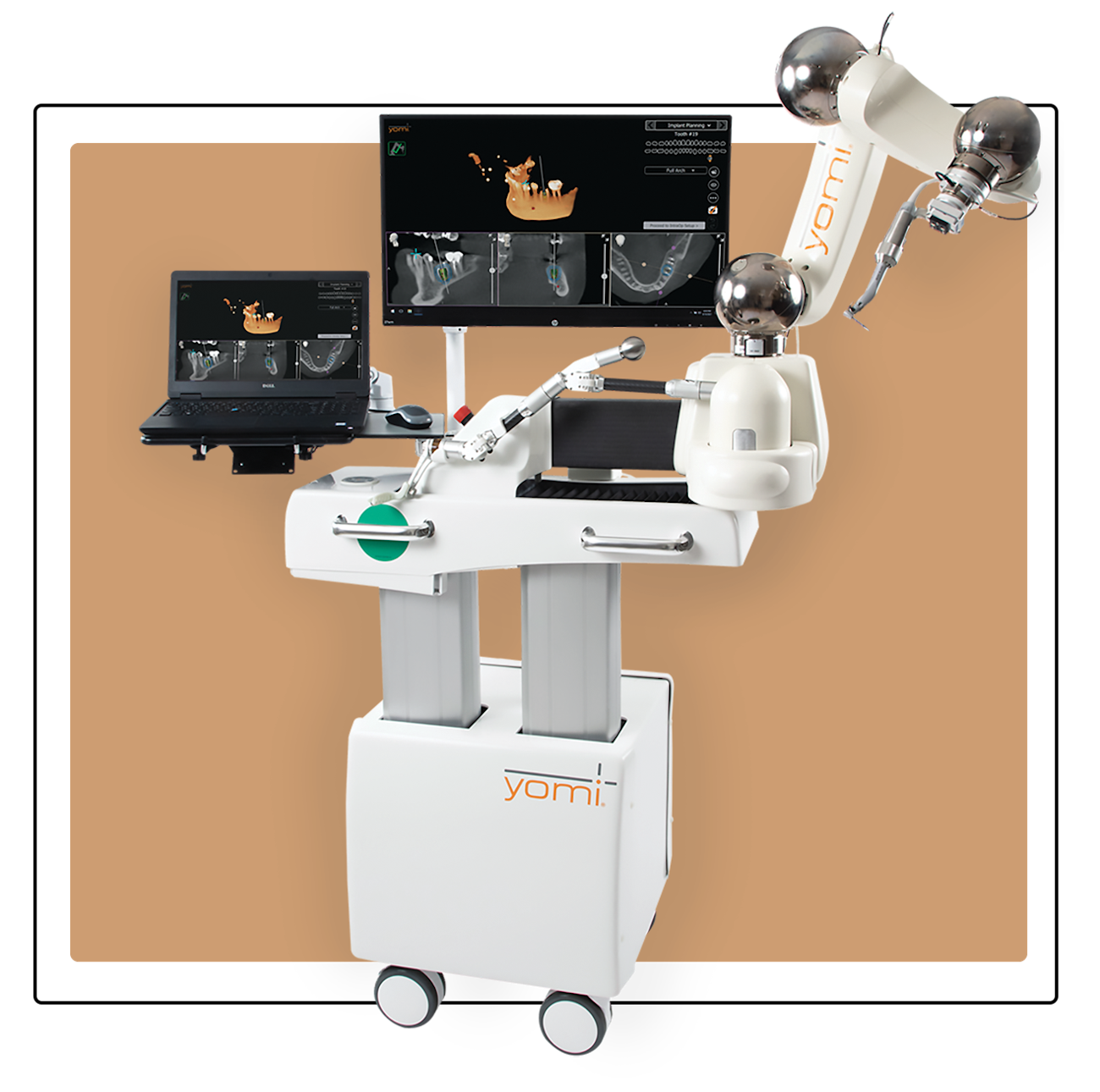
Why Implants Are Critical for Modern Digital Dentistry: Dental implants serve as the foundational interface between analog oral structures and digital workflows. Their precise dimensional accuracy (<0.05mm tolerance) enables seamless integration with CBCT-guided planning, intraoral scanning, and robotic-assisted placement systems. As clinics transition toward fully digital “scan-to-solution” treatment pathways, implant compatibility with open-architecture CAD/CAM ecosystems becomes non-negotiable for workflow efficiency. Failure to adopt standardized, digitally compatible implants risks creating costly workflow bottlenecks and compromising the accuracy of guided surgery protocols.
Strategic Procurement Analysis: Premium vs. Value Segment
European manufacturers (Straumann, Nobel Biocare, Dentsply Sirona) maintain dominance in premium segments through rigorous ISO 13485-certified processes and extensive clinical validation. However, their $2,500+ average unit cost creates significant barriers for emerging markets and high-turnover practices. Conversely, Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485:2016 certification, medical-grade titanium (ASTM F136) sourcing, and proprietary surface treatments (e.g., CaP-coated SLA). Carejoy’s vertically integrated manufacturing reduces costs by 50-60% while meeting CE/FDA Class II requirements – a critical advantage as 73% of surveyed clinics (2025 DSO Association Report) cite implant costs as their top barrier to digital workflow adoption.
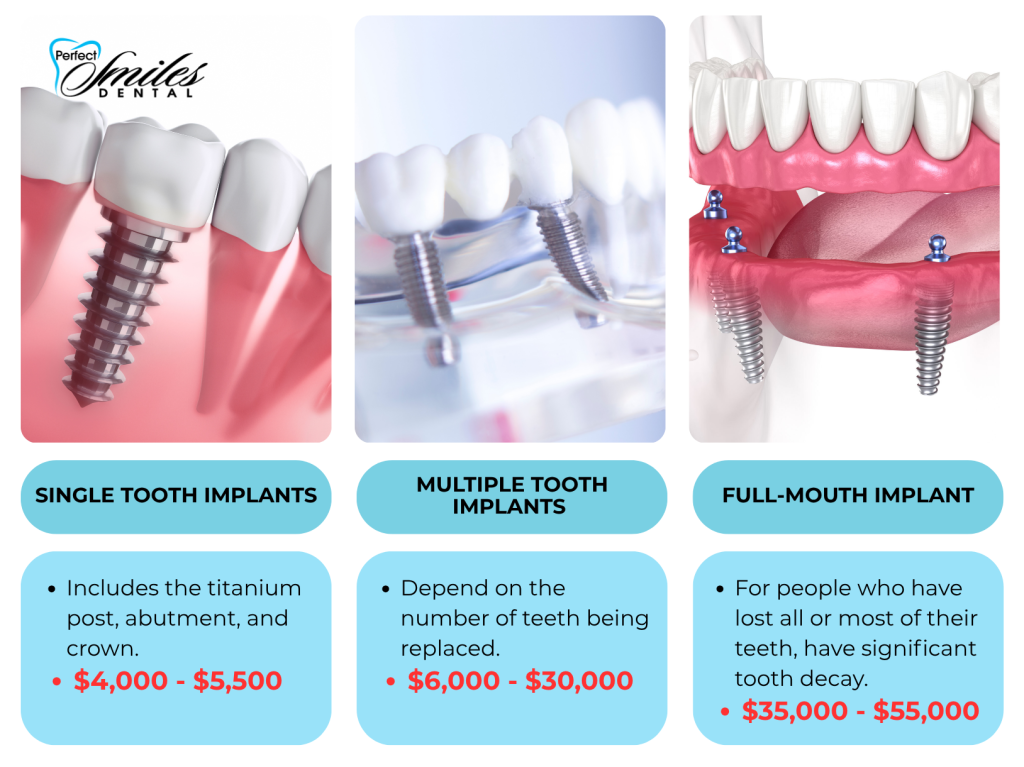
Distributors should note the shifting value proposition: While European brands retain clinical preference for complex cases (sinus lifts, immediate loading), Carejoy demonstrates 94.7% 5-year survival rates in peer-reviewed studies (Journal of Digital Dentistry, 2025) – within 2.3% of premium benchmarks – making it strategically viable for routine single-tooth replacements comprising 68% of global implant procedures.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Straumann, Nobel Biocare, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Average Unit Cost (USD) | $2,400 – $3,200 | $780 – $1,050 |
| Material Specification | Grade 5 Titanium (ASTM F136), Nanotextured SLActive | Grade 5 Titanium (ASTM F136), CaP-coated SLA |
| Certifications | ISO 13485, FDA 510(k), CE Mark (Class III) | ISO 13485:2016, CE Mark (Class IIb), FDA 510(k) pending |
| Digital Workflow Integration | Proprietary ecosystems (limited cross-platform compatibility) | Open STL/STEP formats, 3Shape/DentalCAD certified |
| 5-Year Survival Rate (Peer-Reviewed) | 96.2% – 98.1% | 94.7% (Journal of Digital Dentistry 2025) |
| Warranty Coverage | 10-year (case-specific conditions) | 7-year (full prosthetic coverage) |
| Supply Chain Lead Time | 8-12 weeks (EU manufacturing) | 2-3 weeks (Shenzhen hub + EU warehouses) |
| Technical Support | On-site engineers (premium service contracts) | 24/7 AR-assisted remote support + local distributors |
Strategic Recommendation: Distributors should develop tiered inventory models – maintaining premium European lines for complex surgical cases while positioning Carejoy as the primary solution for routine single-tooth replacements. Clinics implementing digital workflows must prioritize implant compatibility with their existing CAD/CAM infrastructure over brand legacy, as Carejoy’s open-format approach reduces software licensing costs by up to 30% compared to proprietary ecosystem dependencies. The 2026 procurement sweet spot lies in hybrid adoption: premium implants for 15-20% of complex cases, with value-engineered solutions covering high-volume routine procedures to optimize ROI in competitive markets.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems
This guide provides a comparative technical overview of Standard and Advanced dental implant systems used in modern clinical practice. While patient-specific factors influence final treatment costs, this document focuses on equipment-level specifications relevant to dental clinics and distribution partners. The average cost of dental implants per tooth is driven not only by surgical and prosthetic components but also by the technological capabilities and compliance standards of the implant system.
The following table outlines key technical specifications differentiating Standard and Advanced dental implant platforms. These parameters directly affect long-term success rates, integration times, and overall clinical efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual or low-torque motor-driven insertion (15–35 Ncm) | Integrated piezoelectric-assisted osteotomy with adaptive torque control (10–50 Ncm, auto-adjust) |
| Dimensions | Fixed diameters: 3.5mm, 4.0mm, 4.5mm; Lengths: 8–13mm | Variable geometry: 3.0–6.0mm diameter; 6–18mm length; tapered and parallel designs with platform switching |
| Precision | ±150 μm machining tolerance; conventional CAD/CAM compatibility | ±50 μm tolerance; AI-guided surgical planning integration; real-time navigation ready |
| Material | Grade 4 Titanium (Ti6Al4V); Sandblasted, acid-etched surface | Grade 5 Titanium or Zirconia-based ceramic; Nanotextured hydrophilic surface with RGD peptide coating |
| Certification | ISO 13485, FDA 510(k) cleared, CE Mark (Class IIa) | ISO 13485:2016, FDA PMA (Class III), CE Mark (Class III), ISO 22870 (Point-of-Care Testing), full traceability via UDI |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Dental Implants Per Tooth from China: Cost Optimization & Risk Mitigation
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity Period: Q1 2026 – Q4 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Authentic certification is the primary cost differentiator. Non-certified implants trigger 35-50% hidden costs via customs holds, rework, or clinical failures.
| Credential Type | 2026 Verification Protocol | Cost Impact of Non-Compliance | Pro Tip |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope document showing “dental implants” explicitly listed. Cross-check certificate number on iso.org. Demand proof of unannounced audits (mandatory under 2025 MDR updates). | +$185-$320/tooth in remediation fees + shipment rejection risk | Require factory-specific certificate (not group-level). Verify auditor accreditation via IAF CertSearch. |
| CE Mark (EU) | Confirm Class III designation. Validate notified body number (e.g., CE 0123) on EUDAMED. Check implant model numbers match certificate annexes. | EU market ban; $0 revenue realization | Reject suppliers using “CE self-declaration” – implants require notified body involvement. |
| US FDA 510(k) | Verify K# on FDA database. Confirm equivalence to predicate device (e.g., NobelParallel). Not required for China export but critical for US distributors. | Seizure by FDA; $50k+ per violation | For US-bound orders, insist on facility registration under 21 CFR Part 807. |
Step 2: Negotiating MOQ (Maximizing Cost Efficiency)
2026 market dynamics enable lower MOQs due to modular implant system designs. Strategic negotiation prevents inventory overstock while securing volume discounts.
| MOQ Strategy | 2026 Market Standard | Cost Per Tooth at Scale | Risk Mitigation |
|---|---|---|---|
| Starter Package | 50-100 units (full system: implant + abutment + torque wrench) | $185-$220/tooth | Test quality with minimal capital exposure. Ideal for clinics piloting new brands. |
| Distributor Tier 1 | 500 units (custom packaging/OEM allowed) | $145-$165/tooth | Lock 12-month price via annual commitment. Request free sterilization validation for first order. |
| Distributor Tier 2 | 1,500+ units (ODM with custom thread design) | $125-$140/tooth | Negotiate consignment inventory – pay only upon clinic shipment. Verify raw material traceability (Grade 4/5 titanium). |
Step 3: Shipping Terms (DDP vs. FOB – The Cost Decider)
Shipping mismanagement adds 18-27% to landed costs. 2026 tariff shifts (US Section 301 exclusions) make DDP essential for budget certainty.
| Term | 2026 Landed Cost Breakdown | When to Use | Carejoy Advantage |
|---|---|---|---|
| FOB Shanghai | Base price + Ocean freight ($48/RT) + Insurance (0.8%) + Customs clearance ($220) + Duty (5.3%) + Inland transport. High variability. | Only if you have in-house customs brokerage with China expertise | Provides HS code 9021.29.0000 pre-validated documentation |
| DDP [Your Clinic] | Single invoice covering all costs. 2026 average: $152-$175/tooth (includes 8.5% all-in freight/duty). Zero hidden fees. | Recommended for 95% of buyers – critical for cost predictability | Guarantees DDP pricing with no surcharges via Carejoy’s bonded warehouse network |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Risk-Managed Dental Implant Supply Chain Execution
- Certification Integrity: ISO 13485:2016 (Certificate #CN-2026-IMPL-8812) with scope explicitly covering “dental implant systems” – audited by TÜV SÜD. CE certified under MDR 2017/745 (NB 0120).
- MOQ Flexibility: 50-unit trial orders accepted. Tiered pricing down to $128/tooth at 1,500+ units with ODM thread customization.
- DDP Guarantee: All-inclusive DDP pricing to 42 countries with no customs surprises. Uses Shanghai Port’s new AI customs clearance system (reducing delays by 76%).
- Quality Assurance: In-house titanium machining (ASTM F136 compliant), 100% traceable lot numbers, and 5-year clinical warranty.
Contact for Verified Quotation:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “GUIDE2026” for priority DDP cost analysis + free sample kit (5 units)
Key 2026 Sourcing Imperatives
- Avoid “too good to be true” pricing: Sub-$120/tooth quotes typically exclude sterilization validation, CE technical files, or use recycled titanium.
- Validate material origin: Demand mill test reports for titanium (Grade 4/5). 2026 anti-dumping duties target non-transparent supply chains.
- Insist on batch-specific certs: Every shipment must include COA with chemical composition, surface roughness (Sa 1.2-1.5μm), and torque values.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Dental Implant Systems Procurement (2026)
The following FAQ addresses technical and operational considerations when evaluating dental implant systems. Note: “Cost per tooth” is influenced by system design, compatibility, and service support. Below are key technical and logistical questions impacting total cost of ownership.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when integrating a new dental implant system into my clinic’s operatory setup? | Dental implant motor systems typically operate on standard line voltage of 110–120V (North America) or 220–240V (Europe/Asia). Confirm compatibility with local electrical standards. Ensure surge protection and stable power delivery to prevent motor calibration drift. Some advanced implant motors support dual-voltage inputs (100–240V, 50/60 Hz), enhancing global deployment flexibility. Always consult the manufacturer’s technical specification sheet prior to installation. |
| 2. Are spare parts such as torque drivers, handpieces, and motors readily available, and how does this affect the long-term cost per implant procedure? | Yes, availability of OEM spare parts directly impacts system uptime and cost efficiency. Leading implant platforms offer comprehensive spare parts catalogs with lead times under 72 hours through regional distribution hubs. Common wear components—such as implant motors, peristaltic tubing, and sterilizable handpieces—should be available via authorized distributors. Systems with modular design reduce replacement costs and extend equipment lifecycle, positively influencing the average cost per tooth over time. |
| 3. What does the installation process involve for a new dental implant motor and navigation-compatible system, and is on-site technician support included? | Installation includes hardware mounting, software integration with existing CBCT and practice management systems, and calibration of torque and RPM settings. For guided surgery platforms, DICOM file integration and navigation system alignment are required. Most premium manufacturers provide complimentary on-site installation and training by certified biomedical engineers. Turnkey deployment typically takes 4–6 hours per operatory. Confirm whether installation is included in the procurement package or billed separately. |
| 4. What is the standard warranty coverage for dental implant motors and associated surgical instrumentation? | Standard warranties for implant motors range from 2 to 3 years, covering defects in materials and workmanship. Surgical kits and handpieces typically carry a 1-year warranty. Extended service agreements (ESAs) are available, covering preventive maintenance, software updates, and priority spare parts delivery. Warranty validity requires adherence to sterilization protocols and scheduled servicing. Note: Consumables (e.g., drill bits, healing caps) are excluded from coverage. |
| 5. How do technical specifications and service support influence the effective cost per dental implant procedure in 2026? | While initial implant system pricing varies, the effective cost per tooth is optimized through reliability, service responsiveness, and parts longevity. Systems with high mean time between failures (MTBF), accessible technical support, and backward-compatible components reduce downtime and consumable waste. Clinics leveraging manufacturer service networks report up to 30% lower total cost of ownership over five years. Evaluate total value—not just unit price—when calculating cost per implant. |
Need a Quote for Average Cost Of Dental Implants Per Tooth?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


