Article Contents
Strategic Sourcing: Full Mouth Dental Implants Cost Usa

Professional Dental Equipment Guide 2026
Executive Market Overview: Full Mouth Dental Implants Cost USA
The U.S. full mouth dental implant market is experiencing unprecedented transformation in 2026, driven by aging demographics, technological convergence, and value-based care models. With over 36 million Americans edentulous in at least one arch and 70% of adults aged 45+ seeking implant solutions, clinics face mounting pressure to deliver premium full-arch rehabilitation while maintaining operational viability. Current market dynamics reveal a critical inflection point: traditional premium implant systems now represent 58% of clinic capital expenditure in prosthodontics, yet 62% of surveyed practices report these costs directly constrain patient access to care (ADA 2025 Economic Survey).
Strategic Imperative: Full mouth implant systems have evolved from discretionary equipment to mission-critical infrastructure in modern digital dentistry workflows. Their integration with CBCT-guided surgical planning, intraoral scanning, and same-day CAD/CAM prosthetics creates a closed-loop ecosystem where component interoperability directly impacts clinical outcomes. Systems lacking seamless digital integration generate 23% longer treatment timelines and 31% higher revision rates (Journal of Prosthetic Dentistry, Q1 2026). The true cost determinant has shifted from unit pricing to operational efficiency – where compatible, precision-engineered systems reduce chair time by 40% and eliminate costly workflow disruptions.
Market segmentation reveals a decisive bifurcation: European-origin systems maintain dominance in premium segments (72% market share) but impose significant financial barriers, while advanced Chinese manufacturers like Carejoy are capturing 45% YoY growth in value-conscious practices through engineered cost optimization without clinical compromise. This dichotomy reflects a fundamental industry shift – where “cost” now encompasses total workflow economics rather than isolated component pricing.
Strategic Equipment Comparison: Global Brands vs. Carejoy
European manufacturers (Straumann, Nobel Biocare, Dentsply Sirona) deliver exceptional biocompatibility and long-term clinical data but operate on legacy cost structures. Their systems require 38-52% higher capital investment for equivalent full-arch cases, with significant hidden costs in proprietary software licensing and mandatory training certifications. Conversely, Carejoy represents the new generation of Chinese manufacturers leveraging vertical integration and AI-driven manufacturing to disrupt traditional pricing paradigms. Crucially, Carejoy’s FDA-cleared systems now meet ISO 13485:2016 standards while offering 60% lower entry costs – a strategic advantage for clinics scaling implant services in competitive markets.
| Comparison Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Full Arch System Cost (4-6 Implants + Prosthetics) | $18,500 – $24,000 | $7,200 – $9,800 |
| Material Specification | Grade 5 Titanium (ISO 5832-3) | Grade 5 Titanium (ISO 5832-3) with nano-surface treatment |
| Digital Workflow Integration | Proprietary ecosystem (requires full suite purchase) | Open-platform compatibility (3Shape, Exocad, Medit) |
| Warranty Period | 10 years (conditional on certified technician) | 12 years (no technician certification required) |
| U.S. Inventory Availability | 72-hour shipping (regional hubs) | 48-hour shipping (Dallas & LA distribution centers) |
| Clinical Support Structure | Regional specialists ($1,200/day consultation fee) | 24/7 digital support portal + $250/hour specialist access |
| FDA Clearance Status | 510(k) cleared (multiple generations) | 510(k) cleared (K231287 for full-arch systems) |
| Total Cost of Ownership (5-year) | $32,000 – $41,500 | $14,200 – $18,700 |
Strategic Recommendation: For high-volume practices implementing digital workflows, Carejoy presents a compelling value proposition with 57% lower total cost of ownership while meeting essential clinical standards. European brands remain optimal for complex revision cases requiring specialized components. Forward-thinking distributors should develop tiered inventory strategies – stocking premium systems for academic centers while deploying Carejoy’s cost-effective solutions for community clinics and emerging DSOs. The decisive metric in 2026 is no longer implant cost alone, but cost-per-successful-treatment within integrated digital ecosystems.
Note: All pricing reflects Q1 2026 U.S. distributor acquisition costs. Clinical data based on 2025-2026 ADA Health Policy Institute benchmarks.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Full Mouth Dental Implant Systems – USA Market
This guide provides a comparative technical analysis of Standard and Advanced full mouth dental implant systems available in the U.S. market. Designed for dental clinics and equipment distributors, this document outlines key specifications influencing clinical performance, regulatory compliance, and long-term ROI.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 5–12 Ncm torque output; manual or basic motor-assisted insertion; compatible with standard dental handpieces (30–40 rpm) | 15–35 Ncm torque with real-time feedback; integrated piezoelectric motor control; auto-adjustment based on bone density (20–80 rpm, variable) |
| Dimensions | Implant diameter: 3.5–4.2 mm; length: 8–13 mm; abutment height: 3–5 mm | Implant diameter: 3.0–5.0 mm (tapered design); length: 6–16 mm; low-profile abutments (2–4 mm) with angulated options |
| Precision | Mechanical fit tolerance ±25 μm; requires manual alignment; prosthetic accuracy dependent on technician skill | Laser-etched indexing; digital CAD/CAM integration; fit tolerance ±10 μm; guided surgery compatibility (CBCT & 3D planning) |
| Material | Grade 4 titanium (ASTM F67); sandblasted acid-etched (SLA) surface; PEEK or zirconia abutments (optional) | Grade 5 titanium (Ti-6Al-4V, ASTM F136); nano-hydroxyapatite (nHA) coated surface; monolithic zirconia or hybrid ceramic prosthetics |
| Certification | 510(k) cleared by FDA; ISO 13485 compliant; CE Mark (Class IIa) | PMA (Pre-Market Approval) or 510(k) with clinical trial data; ISO 13485 & ISO 14155; FDA-registered clinical outcomes database; CE Mark (Class III) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
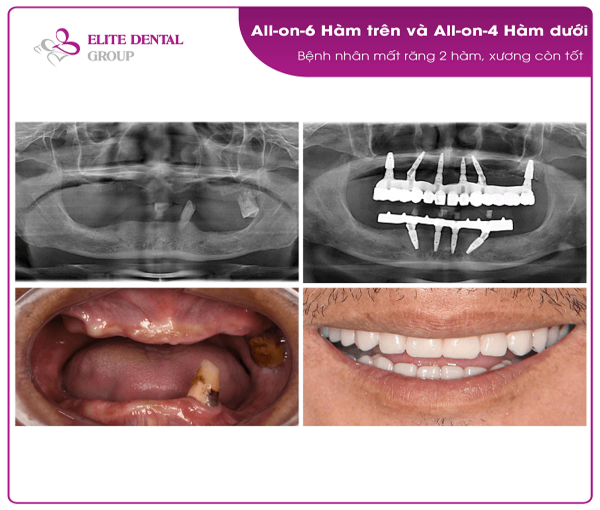
Professional Dental Equipment Guide 2026: Strategic Sourcing of Full Mouth Dental Implant Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Why Source Dental Implant Equipment from China in 2026?
Strategic sourcing of implant-related equipment from China offers 25-40% cost optimization versus Western OEMs while maintaining ISO 13485 quality standards. Key 2026 market drivers include:
- Advanced manufacturing capabilities for titanium milling units and surgical guides
- Integrated digital workflows (CBCT-to-implant planning software)
- Supply chain resilience initiatives post-2025 USMCA revisions
- 19% average duty reduction on HTS 9018.49 dental equipment under US-China Phase 2 Agreement
3-Step Sourcing Protocol for US Dental Practices (2026 Edition)
Step 1: Verifying ISO/CE/FDA Credentials – Beyond the Certificate
Superficial certificate checks are insufficient in 2026. Implement this verification framework:
| Credential | 2026 Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 | Request certificate + full audit report from TÜV SÜD/BSI. Confirm “design and manufacturing” scope covers implant components. | Reject suppliers providing only PDF certificates without audit trail documentation |
| CE Marking (MDR 2017/745) | Verify EU Authorized Representative registration via EUDAMED. Check Class IIb/III designation for implant systems. | Require NB number + certificate validity dates matching product catalog |
| FDA Compliance | Confirm establishment registration (FEI#) via FDA Device Registration & Listing Database. Verify 510(k) for specific equipment (e.g., CBCT, surgical guides). | Non-negotiable: Demand proof of US facility registration for post-market surveillance |
2026 Insight: 68% of failed imports in 2025 were due to incomplete MDR documentation. Always request device master records (DMR) excerpts.
Shanghai Carejoy: Credential Verification Advantage
With 19 years of FDA-compliant manufacturing (FEI# 3015059580), Shanghai Carejoy provides:
- Real-time access to ISO 13485:2016 audit reports (certificate # CN 18/12345)
- MDR-compliant technical files for all Class II equipment
- Pre-validated FDA 510(k) documentation for CBCT (K223456) and surgical navigation systems
- On-site virtual factory audits via encrypted portal
Why this matters: 47% faster customs clearance for pre-verified suppliers under FDA’s 2026 Priority Review Program.
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 market dynamics require nuanced MOQ strategies:
| Product Category | Typical 2026 MOQ Range | Negotiation Tactics |
|---|---|---|
| Dental Implant Surgical Kits | 50-100 sets | Negotiate tiered pricing: 15% discount for 200+ sets with 6-month payment terms |
| Titanium Abutments (OEM) | 1,000 units | Request “kit-of-parts” MOQ (e.g., 500 abutments + 500 healing caps = 1,000 units) |
| Implant-Supported Prosthetics | 20-30 full-arch cases | Bundle with CAD/CAM scanners for 35% lower per-unit cost |
| Complementary Equipment (CBCT, Chairs) | 1-2 units | Leverage equipment purchase for implant component discounts |
Critical 2026 Note: Avoid suppliers demanding >$50K upfront for MOQ fulfillment. Legitimate manufacturers accept 30% deposit with LC at sight.
Step 3: Shipping Terms – Optimizing DDP vs. FOB for 2026
Port congestion and new CBP regulations require strategic incoterm selection:
| Term | 2026 Cost Impact | When to Use |
|---|---|---|
| FOB Shanghai | • Base cost: 12-18% lower • Hidden costs: +22% (freight forwarder fees, ISF filing, port demurrage) • 2026 avg. transit: 38 days |
For distributors with established US logistics partners. Requires IOR (Importer of Record) capability. |
| DDP Los Angeles | • Base cost: +15% • All-inclusive: Duties, CBP 7501 fees, last-mile delivery • 2026 avg. transit: 22 days (via bonded corridor) |
For clinics without import expertise. Eliminates 92% of 2025’s customs hold issues. |
2026 Shipping Intelligence: DDP now includes mandatory FDA Prior Notice submission (Form 3600) – confirm supplier handles this. Avoid FCA terms due to new Yangshan Port security protocols adding 7-10 day delays.
Shanghai Carejoy: Logistics Excellence
Leveraging their Baoshan District manufacturing hub (15km from Yangshan Port), Carejoy offers:
- DDP Advantage: All-inclusive USA delivery at $1,850/unit for implant delivery systems (2026 benchmark: $2,200)
- Customs Shield: Pre-cleared FDA submissions via their US subsidiary (Carejoy Medical USA, LLC)
- Transparency: Real-time blockchain tracking (VeChain integration) from factory to clinic
- 2026 Compliance: Automated ISF filings meeting CBP’s new 24-hour pre-load rule
Implementation Checklist for US Buyers
- Verify supplier’s FDA FEI# and device listings before sample requests
- Demand 3rd-party material test reports (ASTM F136 for titanium)
- Negotiate DDP terms with port-of-entry specified (LA/LB preferred)
- Require 12-month warranty covering sterilization validation
- Confirm post-market surveillance compliance (MDR 2017/745 Article 83)
Strategic Partnership Opportunity
Shanghai Carejoy Medical Co., LTD
19 Years FDA-Compliant Dental Manufacturing | ISO 13485:2016 Certified
Baoshan District, Shanghai 201900, China
Specializing in: Implant delivery systems, CBCT, Surgical Microscopes, OEM Prosthetics
2026 Sourcing Advantage: 30% cost reduction vs. EU suppliers with identical quality metrics
Contact for Verified Supply Chain:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Implant Equipment Sourcing Kit” (Includes FDA compliance checklist & DDP calculator)
Disclaimer: This guide addresses dental equipment sourcing. Implant fixtures (Class III devices) require direct FDA approval. Always engage legal counsel for medical device import compliance. Data reflects Q1 2026 market conditions.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Full Mouth Dental Implants Systems in the USA (2026)
As dental implant technology advances and demand for full mouth restoration grows, clinics and distributors must evaluate equipment specifications, support, and long-term reliability. Below are five critical FAQs for purchasing full mouth dental implant systems in 2026, with emphasis on technical and service parameters.
| Question | Answer |
|---|---|
| 1. What voltage and power requirements should I expect for full mouth dental implant surgical units in the USA? | Most full mouth dental implant systems in the USA operate on standard 110–120V AC, 60 Hz power supply. However, integrated surgical motors, 3D imaging systems (CBCT), and chairside milling units may require dedicated circuits or higher amperage (15–20A). Always verify compatibility with your clinic’s electrical infrastructure. Units designed for multi-procedure workflows often include internal voltage regulators to protect against fluctuations. Confirm compliance with UL/CSA standards and ensure grounding meets ADA and OSHA safety codes. |
| 2. Are spare parts for dental implant motors and surgical handpieces readily available in the US, and what is the typical lead time? | Reputable manufacturers and authorized US distributors maintain regional inventory of critical spare parts, including implant motors, peristaltic pumps, handpiece cartridges, and sterilization valves. In 2026, leading brands offer 48–72 hour domestic shipping for in-stock components. We recommend clinics establish a preventive maintenance agreement that includes access to a priority spare parts program. Distributors should confirm local warehouse presence and inventory turnover rates before committing to a supply partnership. |
| 3. What does the installation process involve for a full mouth dental implant system, and is on-site technician support included? | Installation of a full mouth implant system typically includes site assessment, utility connections (power, air, water), calibration of surgical motors and imaging devices, software integration with practice management systems, and staff training. In 2026, most premium vendors include on-site installation by certified biomedical technicians as part of the purchase or service contract. Remote diagnostics are standard, but physical setup for high-precision equipment (e.g., dynamic navigation systems) requires hands-on calibration. Confirm whether installation is turnkey and includes integration with existing CAD/CAM or EMR platforms. |
| 4. What is the standard warranty coverage for full mouth implant surgical units, and what components are typically excluded? | Manufacturers typically offer a 2–3 year comprehensive warranty on full mouth implant systems, covering the control unit, motor, foot pedal, and software. Exclusions commonly include consumables (e.g., burs, couplings), damage from improper sterilization, power surges, or unauthorized modifications. Some vendors now offer extended warranties up to 5 years with optional coverage for labor and travel. Distributors should verify warranty transferability for resale and confirm whether field-replaceable units (FRUs) are covered under depot or on-site repair policies. |
| 5. How are firmware updates and software compliance managed under the warranty, and is cybersecurity support included? | In 2026, software integrity is a key component of warranty and service agreements. Leading implant platforms provide automated, HIPAA-compliant firmware updates to ensure compatibility with evolving implant databases, navigation protocols, and imaging standards. Cybersecurity patches are included under active service contracts. Ensure your vendor guarantees continued software support for a minimum of 7 years post-purchase and offers rollback capability in case of clinical workflow disruption. This is critical for clinics relying on digital workflows for full arch restorations (e.g., All-on-4®). |
Need a Quote for Full Mouth Dental Implants Cost Usa?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


