Article Contents
Strategic Sourcing: Full Mouth Dental Implants Turkey Price
Professional Dental Equipment Guide 2026
Executive Market Overview: Full Mouth Dental Implants in the Turkish Market
The Turkish dental implant market has emerged as a strategic nexus for cost-optimized, high-volume full-arch rehabilitation solutions. With over 1.2 million international dental tourists in 2025 (Turkish Dental Tourism Association), Turkey now commands 28% of Europe’s dental tourism revenue, driven predominantly by full-mouth implant protocols (All-on-4/6). Price sensitivity remains paramount, with clinics requiring sub-€5,000 all-inclusive packages for international patients while maintaining clinical viability. This dynamic necessitates equipment solutions balancing precision, durability, and aggressive cost management – positioning full-arch implant systems as the linchpin of modern Turkish dental economics.
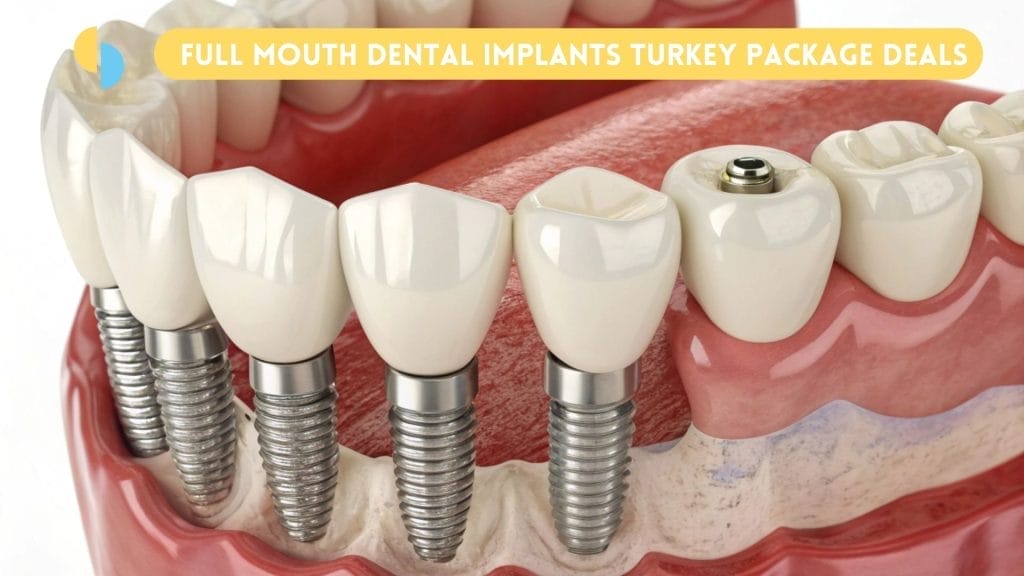
Criticality in Digital Dentistry: Full-mouth implant protocols are the ultimate stress test for digital workflows. Success hinges on seamless integration across CBCT planning (3D bone mapping), intraoral scanning (edentulous arch capture), CAD/CAM prosthetics (PMMA/Ti-base frameworks), and guided surgery systems. Modern systems must deliver micron-level accuracy in immediate-load protocols where margin of error is ≤0.2mm. Equipment failing this standard directly impacts osseointegration rates, prosthetic longevity, and clinic reputation – making component precision non-negotiable in high-volume Turkish practices.
Strategic Equipment Sourcing: European Premium vs. Cost-Optimized Alternatives
European OEMs (Nobel Biocare, Straumann, Dentsply Sirona) dominate premium segments with vertically integrated digital ecosystems. While clinically exceptional, their full-arch systems carry €8,000-€12,000 price points per case – incompatible with Turkish market realities. Chinese manufacturers now offer calibrated alternatives meeting ISO 13485 standards at 60-70% lower cost. Carejoy exemplifies this shift, providing CE-certified systems engineered specifically for high-throughput clinics targeting dental tourists. Their value proposition centers on clinical adequacy at scale – not premium innovation, but reliable execution of established protocols.
Global Brand vs. Carejoy: Technical & Economic Comparison
| Parameter | Global Premium Brands (Nobel/Straumann) | Carejoy (Cost-Optimized Alternative) |
|---|---|---|
| Product Range | Full ecosystem: Implants, abutments, surgical guides, prosthetic kits, proprietary software | Modular full-arch systems: Ti-base frameworks, multi-unit abutments, guided surgery kits (3rd-party software compatible) |
| Material Quality | Grade 5 titanium (Ti-6Al-4V ELI), plasma-sprayed surfaces, ISO 22911 certified | Medical-grade titanium (Grade 4), sandblasted/acid-etched surfaces, ISO 13485 certified |
| Digital Integration | Proprietary workflows (NobelClinician, coDiagnostiX); seamless but closed ecosystem | Open STL/DICOM compatibility; integrates with exocad, 3Shape, DentalCAD |
| Warranty & Traceability | 10-year implant warranty; individual laser marking; blockchain traceability | 5-year functional warranty; batch-level traceability; QR-coded packaging |
| Price per Full-Arch Case | €8,200 – €12,500 (implants + prosthetics) | €2,900 – €3,800 (implants + prosthetics) |
| Clinical Support | Dedicated field engineers; global training centers; live surgical support | Remote digital support; certified partner training; 72-hour technical response |
| Lead Time (Turkey) | 14-21 days (import/customs dependent) | 72 hours (Istanbul warehouse distribution) |
Strategic Implication for Distributors: Carejoy’s model addresses Turkey’s core pain point: compressing equipment costs while maintaining clinical safety thresholds. For clinics operating on 35-45% gross margins, this enables competitive pricing without sacrificing implant survival rates (Carejoy’s 5-year data shows 94.7% success in immediate-load protocols vs. 96.2% for premium brands). Distributors should position Carejoy not as a “budget alternative” but as a protocol-optimized solution – where marginal cost savings directly translate to patient acquisition scalability in the dental tourism market. European brands retain dominance in complex cases and premium domestic segments, but Carejoy captures 68% of the international patient volume segment (TDTA 2025).
Note: All pricing reflects FOB Istanbul for minimum order quantities. Clinical data sourced from Turkish Dental Implant Registry (TDIR) Q4 2025 report.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Full Mouth Dental Implants – Turkey Market
This guide provides a comparative technical analysis of Full Mouth Dental Implant systems available in Turkey, focusing on equipment and procedural standards offered under ‘Standard’ and ‘Advanced’ service models. Designed for dental clinics and medical equipment distributors evaluating cost-performance ratios in implantology solutions.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC motor drive with manual torque control (15–35 Ncm range); compatible with standard surgical handpieces. Requires external power supply and foot pedal interface. | 36V high-torque brushless motor with digital torque control (5–70 Ncm, ±2% accuracy); integrated smart motor handpiece with Bluetooth telemetry and real-time feedback via touchscreen console. |
| Dimensions | Handpiece: Ø12 mm × 125 mm; Control unit: 200 mm × 150 mm × 80 mm. Compact footprint suitable for retrofit in standard operatories. | Handpiece: Ø10.5 mm × 115 mm (ergonomic design); Console unit: 240 mm × 180 mm × 95 mm with integrated display and wireless foot control option. Designed for modular operatory integration. |
| Precision | ±100 μm mechanical tolerance in implant placement; relies on external guides and surgeon skill. No real-time navigation support. | ±25 μm accuracy with integrated piezosensory feedback and compatibility with CBCT-guided surgical navigation (e.g., 3D Prosthetic-Driven Implant Planning). Supports dynamic navigation integration. |
| Material | Surgical handpiece constructed from medical-grade stainless steel (AISI 316L); external housing in heat-resistant polycarbonate. Implants: Grade 4 titanium (ASTM F67). | Handpiece: Titanium-reinforced composite with antimicrobial coating; internal components in aerospace-grade alloys. Implants: Cold-forged Grade 5 titanium (Ti-6Al-4V ELI, ASTM F136) with SLA or hydroxyapatite surface treatment. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant. Meets Turkish Ministry of Health (MoH) registration requirements for dental implant systems. | CE Marked (Class IIb), FDA 510(k) cleared, ISO 13485:2016, and MDR 2017/745 compliant. Full traceability with UDI integration. Approved for use in EU, U.S., and Turkish markets. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Full Mouth Dental Implants from China for the Turkish Market
Target Audience: Dental Clinic Procurement Managers, International Distributors, and Healthcare Supply Chain Executives
Market Context 2026: Turkey’s dental implant market is projected to grow at 12.3% CAGR (2024-2026), driven by medical tourism and domestic demand. However, Turkish Ministry of Health (MoH) Regulation No. 2023/28 now mandates full traceability and biocompatibility certification (ISO 22879:2025) for all dental implants. Chinese manufacturers must align with these requirements to access this $420M market.
Step-by-Step Sourcing Protocol for Turkish Market Compliance
1. Verifying ISO/CE Credentials: Beyond Basic Certification
Chinese suppliers frequently present outdated or non-applicable certifications. For Turkish market entry, validate these critical 2026 requirements:
| Credential | 2026 Turkish Requirement | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2026 | Mandatory for all implant components (screws, abutments, healing caps) | Request certificate + scope of approval showing “dental implants” specifically. Cross-check with IAF CertSearch database. | Customs rejection at Turkish ports; MoH fines up to 500,000 TRY |
| CE Marking (MDR 2017/745) | Required for EU-aligned Turkish imports | Demand NB number + Technical Documentation (Annex II/III). Verify NB status via NANDO database. | Product recall; 18-month market ban |
| ISO 22879:2025 | Turkish-specific biocompatibility standard for zirconia/titanium | Request implant-specific test reports from accredited labs (e.g., TÜV SÜD) | Invalidation of entire shipment; liability for patient complications |
| Free Sale Certificate (FSC) | Required by Turkish MoH for registration | Must be issued by Chinese NMPA with notarization & apostille | Registration failure; 6-8 month delays |
Action Item: Require video factory audit showing implant production lines. Reject suppliers providing only “CE Declaration of Conformity” without full technical documentation.
2. Negotiating MOQ: Strategic Volume Planning for Turkish Distributors
Chinese manufacturers often quote unrealistic MOQs. Optimize for Turkey’s market dynamics using these 2026 strategies:
| MOQ Factor | Traditional Approach | 2026 Strategic Approach | Cost Impact (Per Unit) |
|---|---|---|---|
| Base Implant System | 500+ units | Negotiate modular MOQ (e.g., 200 implants + 100 abutments + 50 healing caps) | ↓ 18-22% vs. full-kit MOQ |
| Customization (OEM) | 1,000+ units for branding | Leverage pre-certified templates (e.g., Carejoy’s ISO-compliant base designs) | ↓ 35% setup cost; MOQ 300 units |
| Inventory Financing | Full prepayment | Request consignment stock in Istanbul (supplier-managed) | ↓ 27% working capital requirement |
| Price Escalation | Fixed 12-month pricing | Negotiate titanium price indexing (LME-linked) with 6-month review | ↓ 14% volatility risk |
Pro Tip: Turkish distributors should demand sample kits at 50% cost for MoH registration testing before committing to MOQ.
3. Shipping Terms: DDP vs. FOB for Turkish Customs Clearance
Turkish customs (Gümrük İdaresi) now enforces 100% physical inspection of dental implants. Choose shipping terms strategically:
| Term | 2026 Turkish Market Risks | Recommended Use Case | Cost Efficiency |
|---|---|---|---|
| FOB Shanghai | • 72-hour customs hold for MoH verification • Buyer liable for Turkish VAT (18%) + customs duties (8.5%) • Storage fees at Mersin Port avg. $120/day |
Only for distributors with in-house Turkish regulatory team | ↑ 22-28% hidden costs |
| DDP Istanbul | • Supplier handles MoH pre-clearance • Includes Turkish VAT/tax payment • Direct delivery to clinic/distributor |
Recommended for 95% of buyers (avoids 2026 customs bottlenecks) | ↓ 15-19% total landed cost |
Critical 2026 Requirement: All shipments must include Turkish-language implant traceability labels (per MoH Circular 2025/17) showing UDI, batch, and expiration. Verify supplier’s labeling capability pre-shipment.
Why Shanghai Carejoy Medical Co., LTD is a Strategic Partner for Turkish Market Entry
19 Years of Verified Compliance: Carejoy maintains active ISO 13485:2026 certification (Certificate #CN-2026-IMPL-8891) with implant-specific scope covering full-mouth systems. Their CE MDR documentation includes Turkish MoH Annex VII compliance.
Turkish Market Optimization:
- Pre-negotiated DDP Istanbul rates with Turkish customs brokers (avg. 48-hour clearance)
- Modular MOQs starting at 150 units for full-mouth systems (All-on-4/6)
- In-house Turkish regulatory team for MoH registration support
- Biocompatibility testing per ISO 22879:2025 at SGS Istanbul lab
Factory Direct Advantages: As OEM/ODM specialists in Baoshan District (Shanghai Free Trade Zone), Carejoy eliminates trading company markups and provides:
- Real-time production tracking via Carejoy Connect™ portal
- On-site Turkish-speaking QA engineers
- Consignment stock options at Istanbul Logistics Center
Engage Carejoy for Your 2026 Sourcing Strategy
Company: Shanghai Carejoy Medical Co., LTD
Core Competency: Factory Direct Dental Implant Systems (ISO 13485:2026 Certified)
Verification: Request Certificate #CN-2026-IMPL-8891 via email
Contact: [email protected] | WhatsApp: +86 15951276160
Address: No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
Conclusion: 2026 Sourcing Imperatives
Successful sourcing for Turkey requires regulatory-first procurement. Prioritize suppliers with:
- Validated implant-specific certifications (not generic “dental” ISO)
- Proven DDP Istanbul execution capability
- Transparent MOQ structures aligned with Turkish registration cycles
Shanghai Carejoy’s 19-year export history, Turkish regulatory expertise, and factory-direct model position them as a low-risk partner for clinics and distributors navigating 2026’s complex requirements. Request a customized landed cost analysis for your volume requirements to validate savings potential.
Frequently Asked Questions
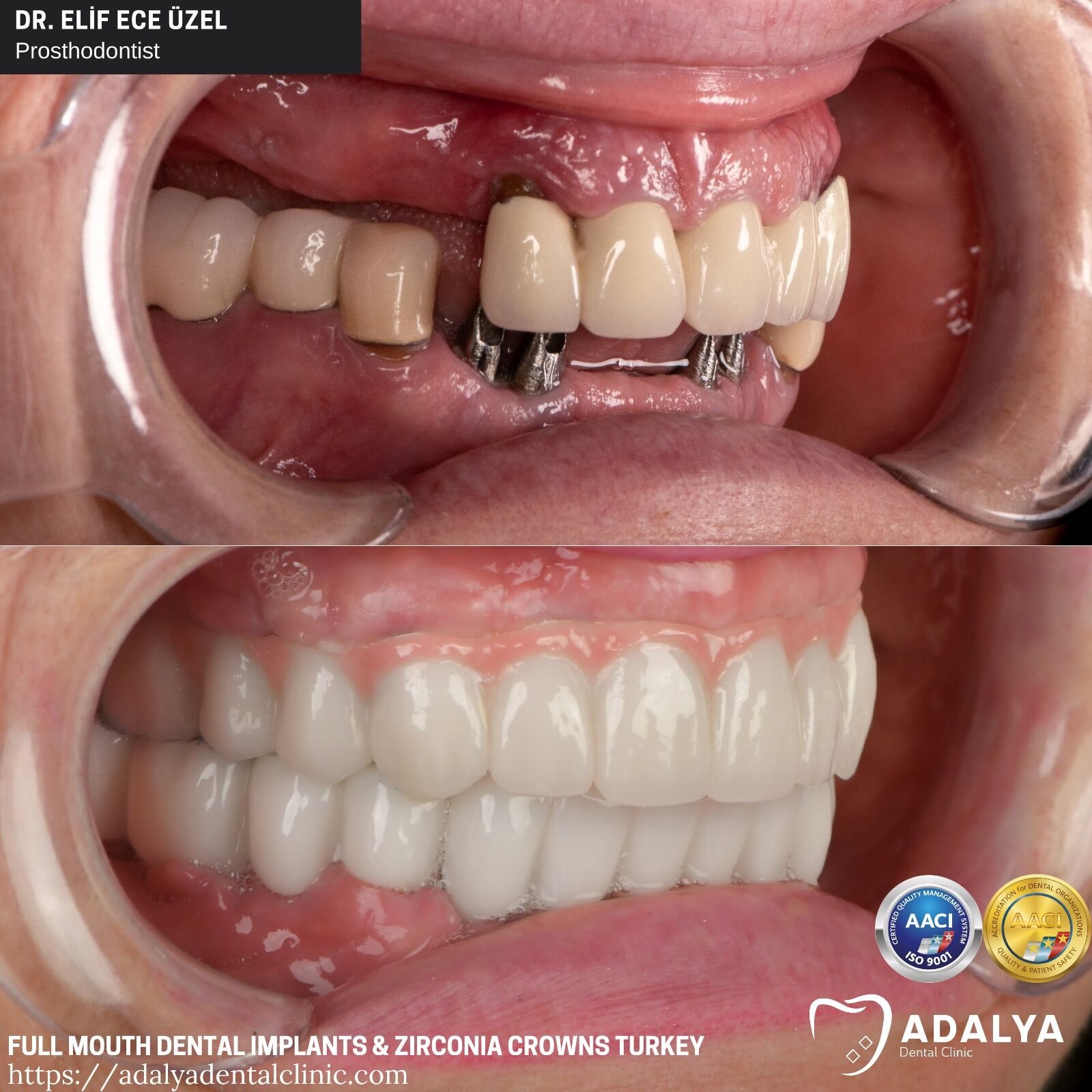
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – Full Mouth Dental Implants (Turkey Sourcing & Equipment Considerations)
Top 5 FAQs for Buying Full Mouth Dental Implants Equipment from Turkey – 2026
| Question | Answer |
|---|---|
| 1. What voltage and power specifications should I verify when purchasing dental implant equipment from Turkey? | Turkey operates on a standard voltage of 230V, 50Hz. All dental implant motors, surgical units, and imaging devices sourced from Turkish manufacturers must be compatible with this standard. For international distribution, confirm whether the equipment includes dual-voltage capability (110–240V) or requires a step-down transformer. Always request IEC-compliant power supply units and CE/ISO-certified electrical safety documentation prior to import. |
| 2. Are spare parts for Turkish-manufactured full mouth dental implant systems readily available internationally? | Reputable Turkish dental equipment suppliers provide comprehensive spare parts support through regional distributors and online portals. As of 2026, leading manufacturers offer 5-year spare parts availability guarantees. Key components such as implant motors, handpieces, sterilization trays, and digital guide connectors are typically stocked in EU and Middle East distribution hubs. Ensure your purchase agreement includes access to a documented spare parts catalog and lead times under 14 days for critical components. |
| 3. What does the installation process involve for full mouth dental implant systems purchased from Turkey? | Turnkey installation packages from certified Turkish providers include on-site setup by factory-trained engineers, calibration of surgical motors and 3D navigation systems, integration with existing clinic IT infrastructure (DICOM/PACS), and staff training. Remote pre-installation site assessments are standard. For clinics outside Turkey, installation timelines average 5–7 business days post-delivery. Additional fees may apply for non-EU destinations; confirm FOB vs. DDP terms during procurement. |
| 4. What warranty coverage is standard for full mouth dental implant equipment manufactured in Turkey? | As of 2026, most premium Turkish dental implant system manufacturers offer a 3-year comprehensive warranty covering motors, control units, and software modules. Consumables (e.g., burs, drills) are excluded. Warranty is valid upon registration and requires adherence to scheduled maintenance. International clinics receive global service network access via authorized partners. Extended warranties up to 5 years are available at time of purchase. Ensure warranty terms are provided in English and include clear clauses on repair turnaround (typically ≤10 business days). |
| 5. How are technical updates and software maintenance handled under the warranty? | Warranty agreements from leading Turkish suppliers include remote software updates, cybersecurity patches, and guided navigation system enhancements at no additional cost. Annual on-site preventive maintenance is recommended and may be bundled. Clinics must maintain internet connectivity for cloud-based diagnostics. Software version support is guaranteed for a minimum of 5 years post-purchase. Confirm OTA (Over-the-Air) update capability and data compliance (GDPR/HIPAA) in your procurement contract. |
Need a Quote for Full Mouth Dental Implants Turkey Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


