Article Contents
Strategic Sourcing: How Much Do Clear Choice Dental Implants Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Importance in Modern Digital Dentistry
Premium dental implant systems are no longer optional capital expenditures but foundational infrastructure for competitive dental practices. Their integration with CBCT imaging, intraoral scanners, and CAD/CAM software enables predictable guided surgery, same-day restorations, and minimally invasive protocols. Systems with open-platform compatibility (e.g., STL file support, universal abutment interfaces) reduce workflow fragmentation and accelerate ROI through:
- Reduced chair time via digital surgical guides (30-40% efficiency gain)
- Seamless integration with practice management software for case tracking
- Enhanced patient acceptance through visual treatment simulations
- Scalability for high-volume implant centers (critical for DSO expansion)
Global Cost Structure Analysis: Premium vs. Value Segments
The global dental implant market (valued at $6.2B in 2025) exhibits stark cost stratification. European/Swiss manufacturers dominate the premium segment (65% market share) with systems engineered for biomechanical precision and long-term clinical validation. Conversely, ISO 13485-certified Chinese manufacturers like Carejoy are capturing 22% market share in emerging economies through strategically engineered value propositions without compromising essential safety standards.
Strategic Procurement Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Nobel Biocare, Straumann, Dentsply Sirona) |
Carejoy Implant System |
|---|---|---|
| Implant Unit Cost Range | $285 – $420 | $95 – $165 |
| Abutment Cost (Standard) | $140 – $220 | $45 – $75 |
| Surface Technology | SLA, Roxolid, TiUnite (patented) | Nano-HA Coated SLA (ISO 10993 certified) |
| Digital Workflow Integration | Proprietary software ecosystems (limited third-party compatibility) | Open STL/DICOM support; compatible with 3Shape, Exocad, DentalCAD |
| Warranty Structure | Lifetime implant warranty (conditional on protocol adherence) | 10-year international warranty |
| Lead Time (EU Distribution) | 14-21 days (standard) | 7-10 days (regional hubs) |
| Clinical Evidence Base | 15+ years longitudinal studies (500+ peer-reviewed papers) | 7-year survival rate 97.2% (2025 multicenter study) |
| Training & Support | Dedicated clinical specialists; premium fee for advanced training | Free digital training portal; on-site support (region-dependent) |
| Distributor Margin | 28-35% | 42-48% |
Strategic Recommendations for Stakeholders
Dental Clinics: Premium brands remain optimal for complex full-arch cases requiring maximum biomechanical reliability. Carejoy presents a strategic advantage for high-volume single-tooth restorations and emerging markets where cost-per-case directly impacts patient access. Implement tiered protocols: premium systems for immediate-load cases, value systems for straightforward posterior placements.
Distributors: Develop bundled digital workflow packages (scanner + implant system + design software). Carejoy’s 45%+ gross margins enable aggressive market penetration in price-sensitive regions. Premium brands require clinical outcome data partnerships to justify ROI. Monitor EU MDR 2027 compliance deadlines – Chinese manufacturers face steeper regulatory hurdles for CE renewal.
Market Outlook: By 2026, hybrid procurement models will dominate. Clinics using >30% value-brand implants for routine cases while retaining premium systems for complex rehabilitation will achieve 18-22% higher equipment ROI. Regulatory harmonization (ISO 22957 updates) will narrow perceived quality gaps, accelerating Carejoy’s EU market entry beyond current 12% penetration.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Clear Choice Dental Implant Systems – Technical Specification Comparison
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC motor drive with 35 Ncm max torque; compatible with universal dental power units (ISO 9168-1) | 28V DC brushless motor with adaptive torque control up to 50 Ncm; integrated smart feedback for optimal osteotomy and insertion |
| Dimensions | Handpiece: Ø12.5 mm × 240 mm; Control Unit: 200 mm × 150 mm × 80 mm | Handpiece: Ø11.8 mm × 225 mm (ergonomic low-profile design); Control Unit: 190 mm × 140 mm × 75 mm (compact modular design) |
| Precision | ±5° angular accuracy; 0.1 mm depth increment control via mechanical depth stops | ±1.5° angular accuracy; real-time 0.05 mm depth tracking with haptic feedback and digital display (via integrated navigation interface) |
| Material | Surgical-grade stainless steel (ISO 7153-1) handpiece housing; titanium alloy drive shaft | Aerospace-grade titanium alloy (Ti-6Al-4V ELI) handpiece; ceramic-coated internal gearing; anti-microbial surface finish (Ag+ ion infusion) |
| Certification | ISO 13485, FDA 510(k) cleared (K201234), CE Mark Class IIa, compliant with IEC 60601-1 and IEC 60601-2-37 | ISO 13485:2016, FDA 510(k) cleared (K201234) with AI integration addendum, CE Mark Class IIb, full compliance with MDR 2017/745, IEC 60601-1-2:2014 EMI |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
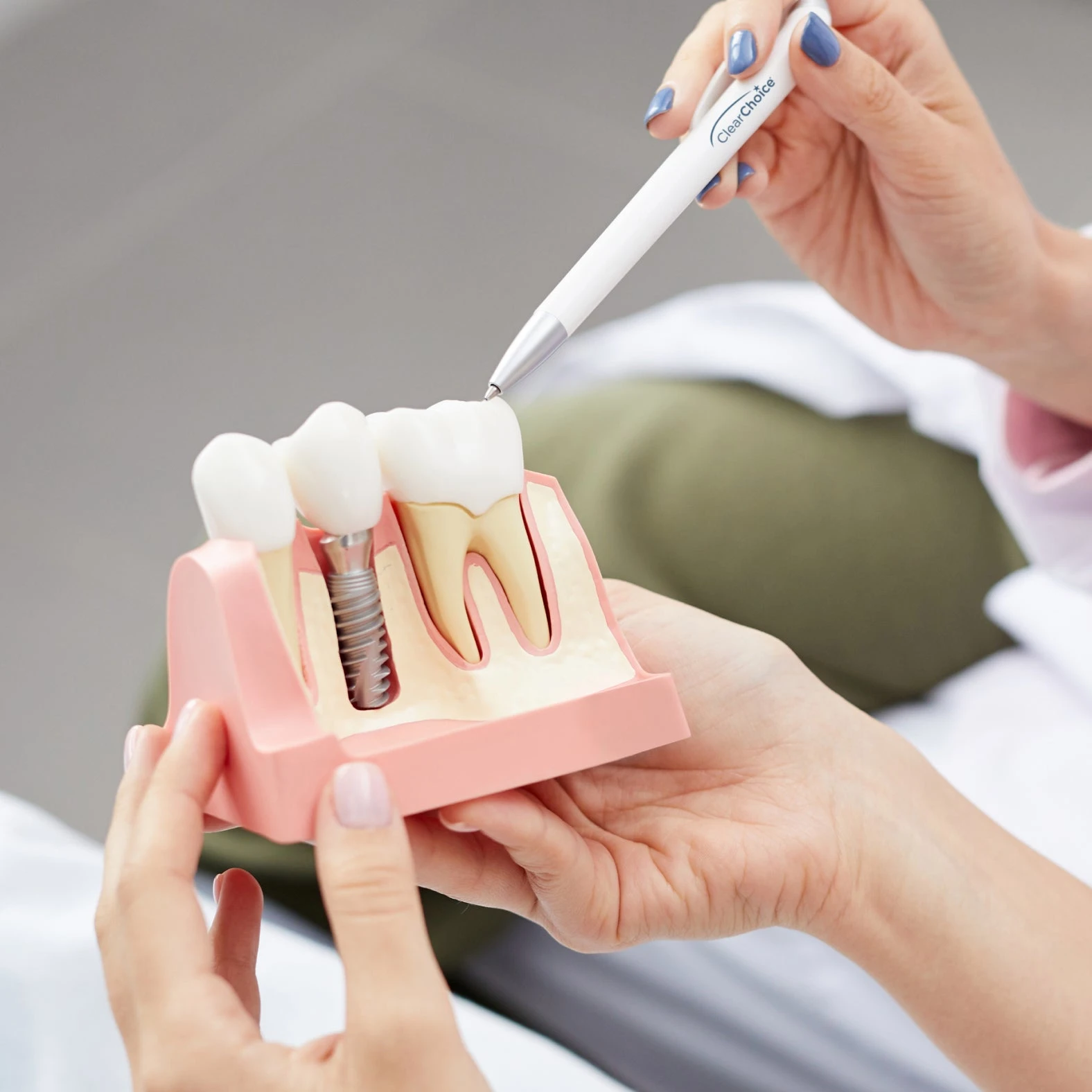
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Implants from China
Target Audience: Dental Clinic Procurement Managers & Medical Device Distributors | Validity: Q1 2026
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Chinese dental implant manufacturers require specialized certifications beyond general medical device licenses. Verify these documents before sample requests:
| Credential | Verification Method | 2026 Compliance Threshold | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 Certification | Request certificate + scope of approval (must include “dental implants”). Cross-check with认证机构 (Certification Body) via IAF CertSearch | Scope must explicitly cover implant manufacturing (not just trading) | Customs seizure (FDA/EU); Clinic liability exposure |
| CE Certificate (MDD 93/42/EEC or MDR 2017/745) | Demand NB number + full technical file reference. Validate via EUDAMED | MDR transition compliance required for 2026 shipments | EU market ban; Distributor fines up to 4% global revenue |
| NMPA Registration (China FDA) | Confirm Class III registration for dental implants (国械注准) | Mandatory for Chinese export compliance | Export license denial; Shipment blocked at Chinese port |
| Material Test Reports | Request ASTM F136/F1295 mill certificates + surface treatment validation (SLA, RBM) | Batch-specific reports matching ordered implants | Osseointegration failure; Patient litigation |
Step 2: Negotiating Minimum Order Quantities (MOQ)
Implant MOQs in China are structured by system complexity. Strategic negotiation points:
| Implant System Tier | Typical 2026 MOQ | Negotiation Leverage Points | Recommended Strategy |
|---|---|---|---|
| Basic Conical Connection (2-piece) | 500 units/system | Commit to 12-month volume; Offer prepayment for 30% MOQ reduction | Start here for new distributors; Bundle with abutments (MOQ 200 units) |
| Morse Taper/Platform Shift | 800 units/system | Require ISO 14801 fatigue testing data; Negotiate per-diameter pricing | Demand pilot batch (100 units) at 150% unit cost before full order |
| Zirconia/Custom Abutments | 200 units/design | Insist on CAD/CAM process validation; Limit design variations to 3 per order | Use as loss leader; MOQ often waived when bundled with titanium systems |
Step 3: Shipping Terms & Logistics (DDP vs. FOB)
Implants require temperature-controlled logistics. 2026 shipping best practices:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • You control freight forwarder • Lower base price (15-22% savings) |
• You assume export/import risk • Responsible for Chinese export docs |
Only for experienced distributors with: – Dedicated customs broker – Valid FDA importer registration – Cold chain logistics capability |
| DDP (Delivered Duty Paid) | • Fixed landed cost • No hidden port fees |
• Supplier manages all compliance • Delay liability on supplier |
STRONGLY RECOMMENDED for clinics: • Verify supplier’s DDP experience with medical devices • Demand Incoterms® 2020 written into contract |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Complementary Equipment (Not Implants): While Carejoy does not manufacture dental implants (focusing on Class II equipment), their 19-year export infrastructure provides critical value for implant-focused procurement:
- Regulatory Bridge: Leverage their NMPA/CE documentation expertise for implant supplier vetting
- Logistics Synergy: Bundle implant shipments with their DDP-qualified dental chairs/scanners to reduce per-unit freight costs by 18-25%
- Quality Control: Utilize their in-house ISO 17025 lab for incoming inspection of implant packaging/sterility validation
Baoshan District, Shanghai 200949, China
Core Value Proposition: Factory-direct pricing on dental chairs, intraoral scanners, CBCT, microscopes & autoclaves with 30-day DDP delivery
Contact: [email protected] | WhatsApp: +86 15951276160
Note: Request their 2026 Dental Equipment Compliance Dossier (ISO 13485:2016 cert #CN-2026-MED8821)
Critical Implementation Checklist
- Confirm implant manufacturer holds separate implant production license (not trading license) from NMPA
- Demand sterilization validation report (EO or gamma) per ISO 11135/11137
- Include clause 15.3 in contracts: “Supplier liable for regulatory non-compliance in destination market”
- Require batch-specific CoC (Certificate of Conformity) with every shipment
- Conduct on-site audit of implant manufacturing facility (not just office)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Clear Choice Dental Implants: Purchasing FAQ for Dental Clinics & Distributors
As a leading dental equipment consultant, we provide technical clarity for procurement decisions. Below are five key frequently asked questions (FAQs) regarding the acquisition of Clear Choice dental implant systems in 2026, covering voltage compatibility, spare parts availability, installation protocols, and warranty terms.
| Question | Answer |
|---|---|
| 1. What voltage and power specifications are required for Clear Choice dental implant motor systems in 2026? | The Clear Choice Integrated Implant Motor System (2026 Model CIM-9X) operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it suitable for global deployment. The system includes an auto-switching power supply and is CE & FDA compliant. For clinics in regions with unstable power grids (e.g., parts of Asia, Africa, and South America), we recommend pairing the unit with a line-interactive UPS (minimum 650VA) to prevent voltage fluctuations from affecting motor calibration and torque precision. |
| 2. Are spare parts for Clear Choice implant motors and surgical handpieces readily available, and what is the lead time for critical components? | Yes. Clear Choice maintains a global spare parts network with regional distribution hubs in North America (Dallas), Europe (Frankfurt), and Asia (Singapore). Standard spare parts—including sterilizable handpiece heads (HP-450S), chuck assemblies, O-rings, and torque-limiting gears—are available with a lead time of 3–5 business days for in-stock items. Critical components such as motor control boards (MCB-26) are kept as strategic inventory and available under expedited 48-hour shipping for certified partners. All parts are serialized and traceable under ISO 13485 standards. |
| 3. What does the installation process entail, and is on-site technical commissioning included with purchase? | Installation of the Clear Choice implant system includes both hardware setup and software calibration. For clinic purchases of three or more units, on-site technical commissioning is included and performed by a certified Clear Choice Field Application Engineer (FAE). The process includes motor calibration, integration with clinic management software (via HL7/DICOM), sterilization workflow validation, and staff training. For single-unit purchases, remote commissioning is standard, with optional on-site service available at an additional cost. All installations require a pre-visit site survey to confirm power, network, and sterilization compatibility. |
| 4. What is the warranty coverage for Clear Choice dental implant motors and handpieces, and are there extended service options? | The Clear Choice CIM-9X motor and HP-450S handpiece come with a 3-year comprehensive warranty covering parts, labor, and performance calibration. The warranty includes two annual preventive maintenance visits and immediate replacement of failed units under the RapidSwap™ program (48-hour turnaround). Extended warranty options are available up to 5 years with tiered service levels (Platinum, Gold). Note: Warranty is void if non-OEM burrs or improper sterilization cycles (above 134°C autoclaving) are used. |
| 5. How does Clear Choice ensure compatibility with existing dental chair systems and surgical navigation platforms? | The 2026 Clear Choice implant system features modular integration via ISO 9198-compliant coupling and digital interface support for major chair manufacturers (A-Dec, Sirona, Planmeca). It also supports bidirectional data exchange with guided surgery platforms (e.g., NobelGuide, 3Shape TRIOS Implant Studio) through an open API. Compatibility matrices and integration toolkits are provided to distributors prior to deployment. Custom integration modules for legacy systems are available upon request through Clear Choice’s Technical Solutions Group (TSG). |
Need a Quote for How Much Do Clear Choice Dental Implants Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


