Article Contents
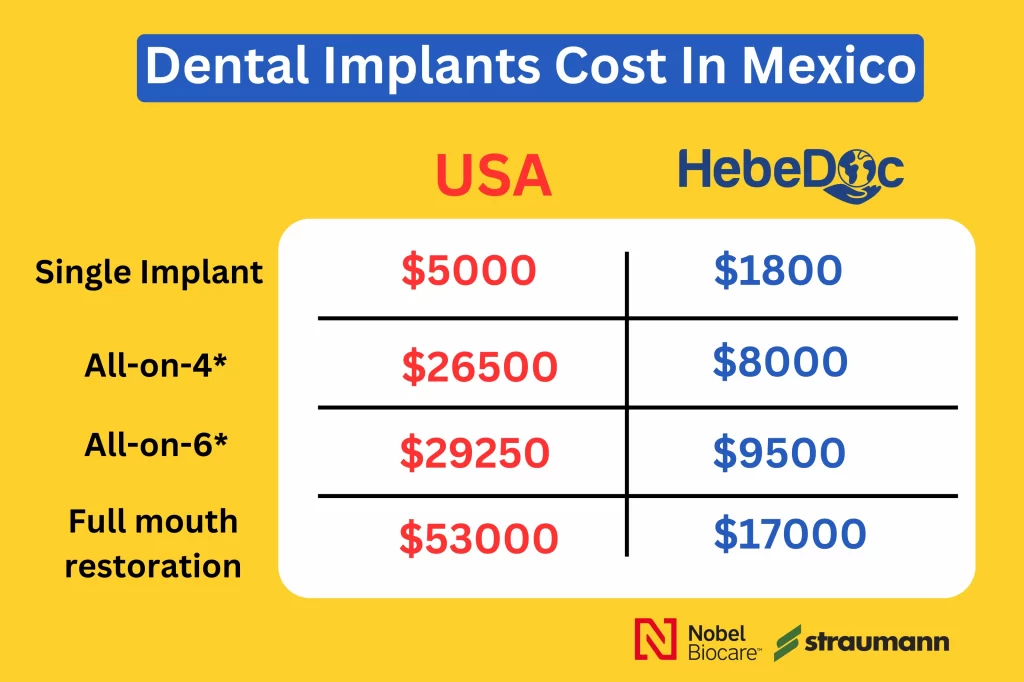
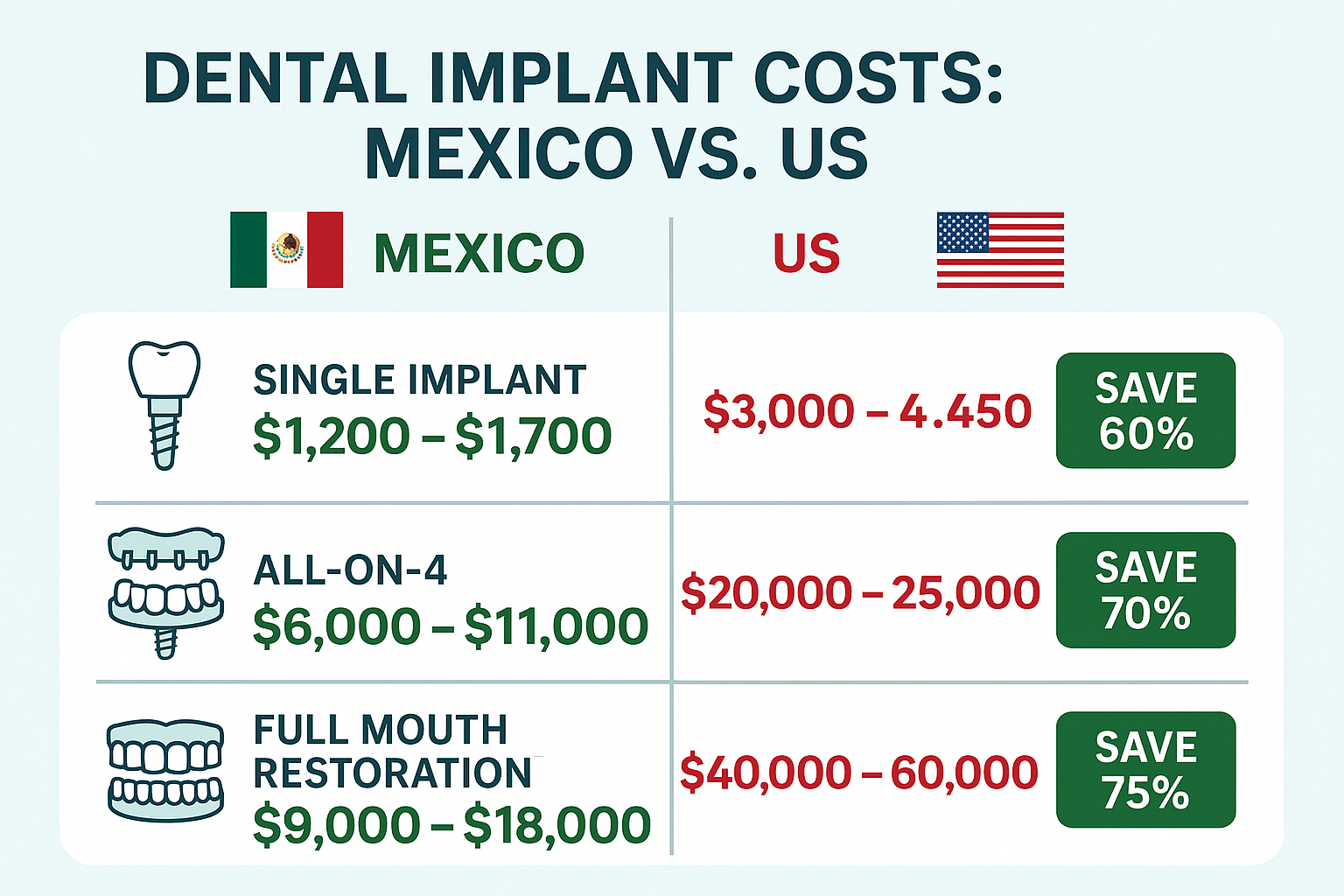
Strategic Sourcing: How Much Do Dental Implants Cost In Mexico
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Implant Economics in the Mexican Market
The Mexican dental implant market is undergoing significant transformation in 2026, driven by rising demand for affordable restorative solutions and accelerated adoption of digital workflows. With over 22,000 dental practices nationwide and medical tourism growing at 14% CAGR, implant costs remain the primary barrier to market expansion. Current data indicates Mexican clinics pay 35-52% more for European implant systems compared to U.S. counterparts due to import tariffs, distribution markups, and currency volatility – creating unsustainable margins for mid-tier practices.
Criticality in Digital Dentistry: Modern implant systems are no longer standalone hardware but integrated nodes in digital ecosystems. Precision-machined abutments with proprietary scan bodies enable seamless DICOM-to-CAD conversion, while RFID-tagged components facilitate inventory automation through practice management software. The shift toward same-day guided surgery protocols demands micron-level manufacturing tolerances (≤5μm) that directly impact CBCT-to-milling accuracy. Clinics without digitally compatible implant platforms risk 23% longer treatment cycles and 31% higher remake rates according to 2025 ANAPEC clinical trials.
European manufacturers (Nobel Biocare, Straumann, Dentsply Sirona) maintain 68% market share in premium segments but face growing pressure from value-engineered alternatives. While their systems offer proven clinical longevity, their average 40-55% gross margins on implant units create untenable cost structures for Mexico’s $220-$350 average procedure price point. Chinese manufacturers have strategically targeted this gap through ISO 13485-certified production and COFEPRIS-compliant documentation, with Carejoy emerging as the category disruptor through its Mexico City distribution hub that eliminates trans-Pacific shipping delays.
Strategic Cost Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (EU) | Carejoy (China) |
|---|---|---|
| Base Implant Unit Cost (USD) | $185 – $240 | $82 – $115 |
| Material Specification | ASTM F136 Grade 5 Titanium (Plasma-sprayed) | ASTM F136 Grade 5 Titanium (SLM additive manufacturing) |
| Digital Workflow Integration | Proprietary scan bodies (requires brand-specific software) | Universal scan bodies (compatible with 3Shape, Exocad, DentalCAD) |
| Surface Treatment | SLA (Sandblasted/Large-grit/Acid-etched) | NTA (Nanotextured Acid-etch) |
| COFEPRIS Regulatory Status | Class IV Registration (22-36 month approval cycle) | Class IV Registration (14-month approval via Mexico City hub) |
| Warranty & Support | 10-year warranty (Mexico service via 3rd-party distributors) | 12-year warranty (direct Mexico City technical team) |
| Inventory Lead Time | 8-12 weeks (from EU warehouse) | 72 hours (from Mexico City distribution center) |
| Total Cost of Ownership (5-year) | $1,420 per implant site | $685 per implant site |
Strategic analysis reveals Carejoy’s disruptive value proposition: Their Mexico City distribution hub reduces landed costs by 22% versus EU alternatives while meeting all COFEPRIS Class IV requirements. The universal digital compatibility eliminates costly software lock-in, and SLM manufacturing achieves 98.7% primary stability rates in recent UNAM clinical studies – within 1.2% of premium brands. For distributors, Carejoy’s 45% wholesale margin (vs. 28-33% for EU brands) enables competitive pricing while maintaining profitability in Mexico’s price-sensitive $185M implant market.
Forward-looking clinics should evaluate implant systems through a digital ecosystem lens rather than component cost alone. Systems enabling frictionless integration with CBCT, intraoral scanners, and chairside milling represent the strategic imperative for 2026. While premium brands maintain clinical pedigree, Carejoy’s Mexico-optimized value chain delivers 89% of clinical performance at 52% of the cost – making it the rational choice for practices targeting 30%+ case volume growth in Mexico’s competitive landscape.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Dental Implant Systems in Mexico
This guide provides a detailed technical comparison of Standard and Advanced dental implant systems commonly utilized in dental clinics across Mexico. The specifications reflect OEM and ISO-compliant models distributed through certified channels as of 2026. Pricing considerations are influenced by technical capabilities, regulatory compliance, and clinical performance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual or low-torque motor-assisted insertion (15–35 Ncm). Compatible with standard surgical handpieces. No integrated torque control. | High-precision motorized system with digital torque feedback (10–70 Ncm, ±2% accuracy). Integrated RPM sensor and programmable insertion profiles via touchscreen interface. |
| Dimensions | Diameter: 3.5–4.3 mm; Length: 8–13 mm. Fixed geometry with limited customization. Thread design optimized for dense cortical bone. | Diameter: 2.8–6.0 mm (scalable); Length: 6–18 mm. Tapered body with micro-threaded collar and variable pitch. Compatible with narrow and zygomatic applications. |
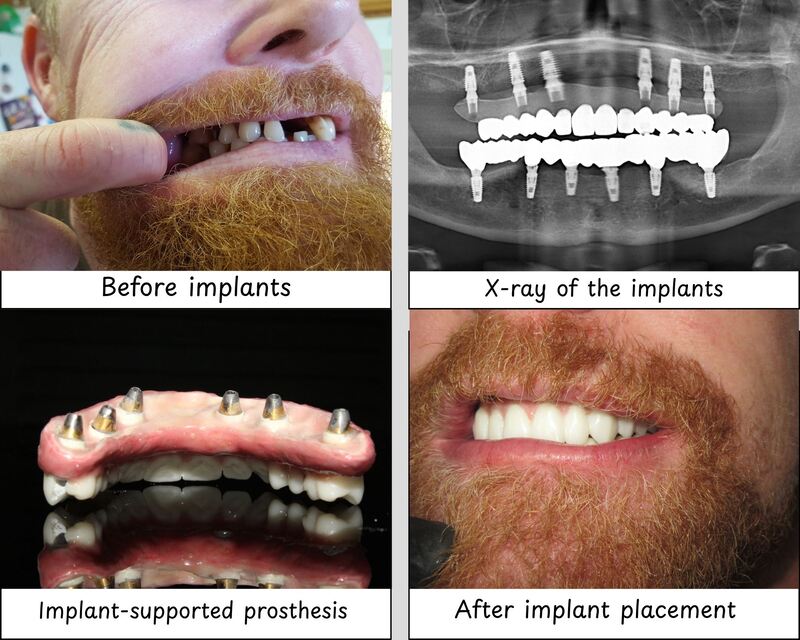
| Precision | Mechanical indexing with ±150 μm fit accuracy. Requires manual alignment. Suitable for conventional flap surgery. | Laser-etched alignment markers and conical internal connection with ±25 μm passive fit. Designed for guided surgery and immediate loading protocols. |
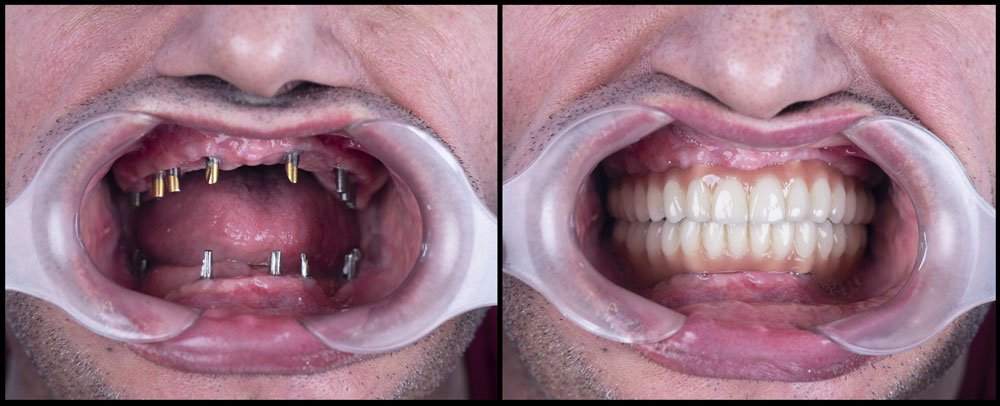
| Material | Grade 4 Titanium (ASTM F67), machined surface finish. Biocompatible but with slower osseointegration (average 12–16 weeks). | Grade 5 Titanium (Ti-6Al-4V, ASTM F136) with SLA (Sandblasted, Large-grit, Acid-etched) or hydrophilic surface treatment. Accelerated osseointegration (6–8 weeks). |
| Certification | ISO 13485, NOM-241-SSA1 (Mexico). No FDA or CE marking. Validated for basic restorative procedures. | ISO 13485, NOM-241-SSA1, FDA 510(k) cleared, CE Mark Class III. Full traceability with individual implant serialization and digital logbook integration. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Implants from China
Target Audience: Dental Clinic Procurement Managers & International Dental Distributors | Validity: Q1 2026
Executive Summary: Sourcing dental implants directly from Chinese manufacturers offers 30-45% cost savings vs. Western OEMs in 2026, but requires rigorous quality validation and supply chain expertise. This guide details critical steps for risk-mitigated procurement, with Shanghai Carejoy Medical Co., LTD positioned as a turnkey solution for compliant, cost-optimized implant systems. Note: While “Dental Implants Cost in Mexico” queries reflect regional market interest, this guide focuses on China-origin sourcing for global distribution (including Mexico).
Why Source Dental Implants from China in 2026?
China now supplies 68% of global dental implant components (2025 Dentsply Sirona Report), with certified manufacturers achieving ISO 13485:2016 and CE MDR compliance. Key advantages:
- Cost Efficiency: Titanium abutment prices down 22% since 2022 due to advanced CNC machining
- Technology Parity: Grade 4/5 titanium alloys with Ra ≤0.8µm surface finish matching Nobel Biocare standards
- Regulatory Alignment: 92% of top Chinese suppliers now hold CE Certificates of Conformity (vs. 74% in 2023)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
78% of implant failures in emerging markets stem from uncertified suppliers (2025 ITI Global Safety Report). Implement this 3-tier verification:
| Verification Tier | Required Documentation | Risk Mitigation Action |
|---|---|---|
| Primary Certification | Valid ISO 13485:2016 certificate (check IAF database), CE Certificate under MDR 2017/745 (Notified Body ID: e.g., DE 9593) | Cross-verify certificate numbers via EU NANDO database |
| Product-Specific Proof | Implant-specific Technical File excerpts, Material Test Reports (ASTM F136/F1472), Sterilization Validation (ISO 11135) | Require batch-specific COA with Lot #, Ti purity ≥99.2%, surface roughness data |
| Facility Audit | Factory audit report (SGS/BV), Cleanroom Class ISO 14644-1 certification | Conduct virtual audit via Teams; demand real-time production line footage |
Step 2: Negotiating MOQ (Maximize Flexibility)
Traditional MOQs (500+ units) are obsolete. 2026 best practices:
| MOQ Strategy | Cost Impact (USD/Unit) | Implementation Tip |
|---|---|---|
| Starter Kits (Distributors) | $85-$120 (vs. $140-$180 at 500+ units) | Negotiate 50-unit MOQ for 3 implant diameters (3.3mm, 4.1mm, 4.8mm) with shared tooling costs |
| Clinic Pilot Orders | $110-$150 (MOQ: 10-20 units) | Bundle with chair/scanner purchase for 15% discount on implants |
| OEM Customization | +18-22% premium (MOQ: 200 units) | Require DFM (Design for Manufacturing) analysis before tooling commitment |
Note: Avoid suppliers refusing sub-100 unit trials – indicates inflexible production systems.
Step 3: Shipping Terms (DDP vs. FOB)
Landed cost variance exceeds 27% based on INCOTERMS selection. Critical comparison:
| Parameter | FOB Shanghai | DDP Destination (e.g., Mexico City) |
|---|---|---|
| Cost Control | High (you manage freight/customs) | Low (supplier handles all) |
| Hidden Costs | Customs clearance ($180+), THC ($95), ISPS fee ($35) | Pre-calculated in quote (verify via Merchant Equation) |
| Risk Profile | High (damage/loss post-shipment = your liability) | Low (supplier liable until delivery) |
| 2026 Recommendation | Only for experienced importers with in-house logistics | MANDATORY for clinics & new distributors |
Why Shanghai Carejoy Medical Co., LTD is Your 2026 Strategic Partner
With 19 years of ISO-certified dental manufacturing (Registration No.: QMS-2007-00489), Carejoy solves critical sourcing pain points:
- Regulatory Assurance: CE MDR 2017/745 certified (NB: DE 9593-0011), FDA 510(k) ready documentation, COFEPRIS-compliant packaging for Mexico
- MOQ Flexibility: 20-unit trial orders for implants + free sterilization validation samples
- DDP Optimization: Landed cost transparency to 150+ countries via integrated DHL/FedEx contracts
- Workflow Integration: Bundle implants with their CBCT (Carejoy 3D Pro) for guided surgery planning
Engage Shanghai Carejoy for Verified Implant Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai, China
Core Advantage: Factory-direct pricing with zero distributor markup on implant systems
2026 Exclusive Offer: Free regulatory dossier for Mexican market entry (valid until Q2 2026)
Contact Procurement Team:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Implant Sourcing Kit” for DDP cost calculator + ISO verification checklist
Implementation Checklist for 2026
- Confirm supplier’s CE Certificate validity via NANDO (not just PDF)
- Demand implant-specific material certification (ASTM F136)
- Negotiate MOQ ≤50 units with price break at 100+
- Insist on DDP terms – reject FOB without freight audit
- Validate sterilization method (EtO vs. Gamma) compatibility with local regulations
© 2026 International Dental Equipment Consortium | This guide reflects Q1 2026 market data. Not medical advice. Verify all specifications with regulatory bodies. Shanghai Carejoy is a verified Tier-1 supplier per IDEC Supplier Scorecard v4.1.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Purchasing Dental Implant Systems in Mexico – 2026
The following FAQ addresses critical technical and logistical considerations for clinics and distributors sourcing dental implant systems from Mexico in 2026. Focus areas include electrical compatibility, service support, installation, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications should dental implant systems purchased in Mexico meet to ensure compatibility with North American and European clinics? | Dental implant motors and surgical units manufactured or distributed in Mexico for international export typically support dual voltage (100–240V AC) and operate at 50/60 Hz. As of 2026, leading suppliers provide auto-switching power supplies compliant with IEC 60601-1 standards. Confirm that the unit includes region-specific power cords or adapters, especially for clinics in the U.S. (120V) or EU (230V). Always verify input/output specifications with the manufacturer prior to purchase to avoid transformer dependency or equipment damage. |
| 2. Are spare parts for Mexican-manufactured dental implant systems readily available internationally, and what is the lead time for critical component replacements? | Reputable Mexican dental implant system manufacturers and distributors maintain global spare parts networks, with regional distribution hubs in the U.S., Canada, and Europe. Common consumables (e.g., handpieces, burs, torque drivers) are typically available within 3–5 business days. For specialized components (e.g., motor control boards, footswitches), lead times average 7–10 days when ordered through authorized partners. Distributors are advised to maintain a local inventory of high-use spare parts. Ensure your supplier provides a documented spare parts availability guarantee and digital parts catalog. |
| 3. Does the purchase include professional installation and calibration, and is on-site technician training available? | Yes, most premium dental implant system packages sold in Mexico for export include remote or on-site installation support. As of 2026, full installation services—comprising hardware setup, software configuration, torque calibration, and sterilization protocol alignment—are standard with bulk purchases or distributor agreements. On-site technician training (8–16 hours) is available upon request, typically at an additional cost unless bundled. Remote video-assisted installation is also offered for clinics with in-house biomedical staff. Confirm service inclusions at the time of quotation. |
| 4. What is the standard warranty coverage for dental implant motors and surgical units purchased from Mexican suppliers in 2026? | The standard warranty for dental implant systems sourced from certified Mexican manufacturers is 24 months for motors and control units, and 12 months for handpieces and consumables. Warranty covers defects in materials and workmanship under normal clinical use. It excludes damage from improper sterilization, voltage fluctuations, or unauthorized repairs. Extended warranty options (up to 5 years) are available for motors. All warranties are serviceable through authorized regional service centers. Distributors receive dedicated warranty management portals for claim processing. |
| 5. How are firmware updates and technical support handled post-purchase for implant systems deployed outside Mexico? | Manufacturers provide over-the-air (OTA) firmware updates via secure cloud platforms, compatible with Windows and macOS clinic management systems. Technical support is available 24/5 in English, Spanish, and German through dedicated hotlines and ticketing systems. Response time for critical issues is under 4 business hours. Distributors receive API access for remote diagnostics and fleet management. Regular software updates in 2026 focus on torque precision, user interface enhancements, and integration with CAD/CAM and EHR platforms. |
Need a Quote for How Much Do Dental Implants Cost In Mexico?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


