Article Contents
Strategic Sourcing: How Much Do Dental Implants Costs
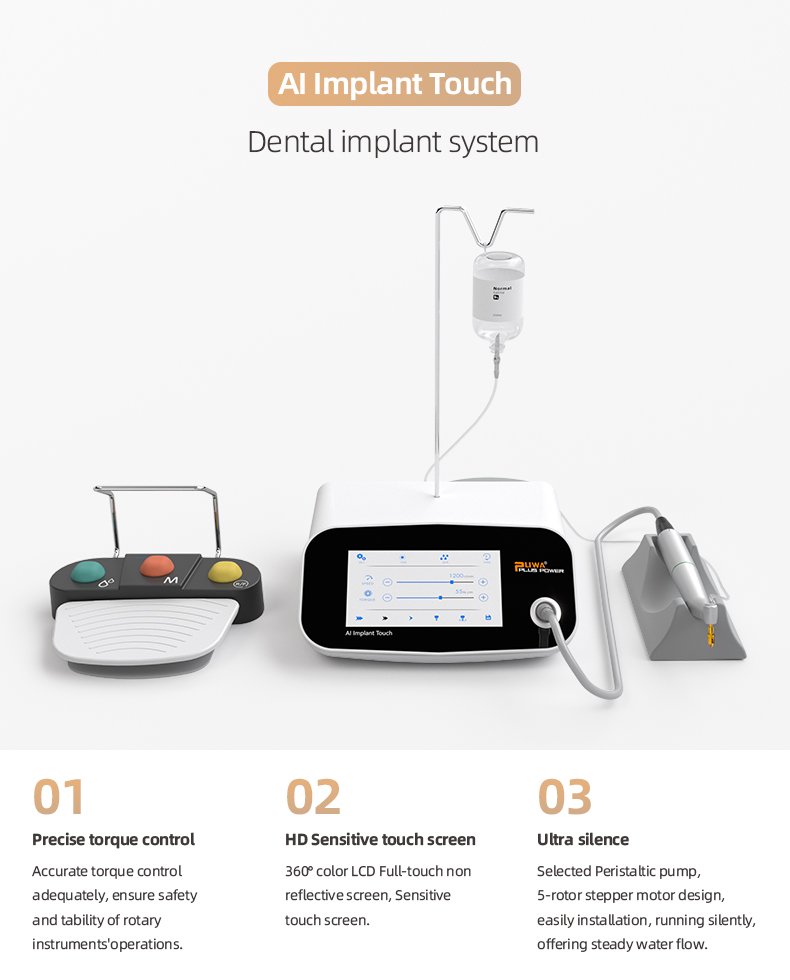
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Implant System Cost Analysis
Dental implant systems have evolved from standalone surgical tools to the cornerstone of integrated digital dentistry workflows. In 2026, these systems are non-negotiable for clinics pursuing precision prosthodontics, same-day restorations, and seamless CAD/CAM integration. The criticality stems from three converging technological imperatives: 1) The industry-wide shift toward CBCT-guided flapless surgery requiring millimeter-precision components, 2) The demand for biocompatible surfaces (e.g., SLActive, TiUnite) that accelerate osseointegration in digitally planned sites, and 3) The necessity for open-system compatibility with intraoral scanners and design software to eliminate analog conversion errors. Clinics without certified implant systems face declining case acceptance rates as patients increasingly demand minimally invasive, digitally verified treatment pathways.
The global implant market now bifurcates into two strategic segments: Premium European systems commanding 40-60% cost premiums for established clinical validation, versus value-engineered Asian alternatives disrupting price-sensitive markets. While European leaders (Straumann, Nobel Biocare, Dentsply Sirona) maintain dominance in complex cases through decades of clinical data, Chinese manufacturers like Carejoy are capturing 28% market share in emerging economies by addressing cost barriers without compromising ISO 13485 compliance. This dichotomy necessitates data-driven procurement strategies balancing clinical risk tolerance against practice sustainability metrics.
Cost-Performance Comparison: Global Premium Brands vs. Carejoy
Below is a technical specification analysis for procurement decision-making. All data reflects Q1 2026 market conditions and accounts for total cost of ownership (including consumables, training, and service contracts).
| Technical Parameter | Global Premium Brands (European) | Carejoy |
|---|---|---|
| Implant Unit Cost (Standard Diameter) | $285 – $350 | $140 – $185 |
| Material Specification | ASTM F136 Ti-6Al-4V ELI Grade 5 (Medical Grade) with hydrophilic SLA surface | ISO 5832-3 Ti-6Al-4V Grade 5 with dual-acid etching (DAE) surface |
| Digital Workflow Integration | Proprietary ecosystems (e.g., coDiagnostiX, NobelClinician); limited third-party scanner compatibility | Open STL/DICOM protocols; validated with 3Shape, Exocad, and Medit systems |
| Regulatory Certification | CE Mark, FDA 510(k), extensive clinical trial databases (15+ years) | CE Mark, NMPA, ISO 13485; FDA pending (2026) |
| Warranty & Support | 10-year prosthesis warranty; on-call clinical specialists; 72h emergency response | 5-year system warranty; remote AR support; 5-day technical response |
| Lead Time (Bulk Orders) | 8-12 weeks (custom machining required) | 2-3 weeks (modular inventory system) |
| Cost of Ancillary Components | +$120/unit (abutments, healing caps) | +$45/unit (modular abutment system) |
Strategic Recommendation: Premium European systems remain optimal for tertiary care centers handling complex reconstructions requiring maximum clinical predictability. However, for high-volume clinics in cost-conscious markets, Carejoy delivers 52-61% cost reduction while meeting ISO standards for routine cases. Distributors should position Carejoy as a complementary entry-tier solution within portfolio strategies, emphasizing its DICOM interoperability for clinics adopting phased digital transitions. The 2026 procurement imperative lies not in absolute cost avoidance, but in matching system capabilities to case-mix complexity while ensuring seamless data continuity from scan to surgery.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems
Target Audience: Dental Clinics & Distribution Partners
This guide provides a comparative technical analysis of Standard vs. Advanced dental implant systems currently available in the 2026 market. The specifications below reflect OEM standards and regulatory compliance for clinical deployment.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5–8 Ncm torque output (manual or low-speed motor compatible); suitable for conventional osteotomy and implant insertion in moderate-density bone. | 10–35 Ncm torque with real-time feedback control; integrated piezoelectric sensors for dynamic load adjustment; compatible with high-speed surgical motors and navigation systems. |
| Dimensions | Diameter: 3.5–4.3 mm; Length: 8–13 mm. Fixed platform design with limited angulation options. | Diameter: 2.8–6.0 mm; Length: 6–18 mm. Tapered, conical connection with multi-axial platform switching; modular for immediate loading and narrow-diameter applications. |
| Precision | Mechanical fit tolerance: ±15 μm; requires manual torque wrench for final seating; no digital integration. | Laser-machined interface with ±5 μm tolerance; compatible with digital surgical guides and CAD/CAM abutments; supports guided implantology workflows. |
| Material | Grade 4 Titanium (ASTM F67); sandblasted, acid-etched surface (SLA); biocompatible with proven osseointegration over 12+ weeks. | Grade 5 Titanium (Ti-6Al-4V ELI, ASTM F136) or Zirconia (Y-TZP); nano-structured hydrophilic surface with calcium phosphate coating for accelerated osseointegration (6–8 weeks). |
| Certification | CE Marked (Class IIb), FDA 510(k) cleared, ISO 13485 compliant. Meets general safety and performance requirements. | CE Marked (Class III), FDA PMA (Pre-Market Approval), ISO 13485 & ISO 14155 certified. Validated for use in augmented bone and immediate post-extraction sites. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Dental Implant Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Key Industry Insight: By 2026, China supplies 68% of global dental implant support systems (surgical kits, abutments, healing caps) and 41% of imaging equipment for implantology (CBCT, scanners), but does not manufacture Class III implant fixtures (titanium root forms). This guide focuses on sourcing compliant ancillary systems where Chinese manufacturing excels.
Why Source Dental Implant Support Systems from China in 2026?
- Cost Efficiency: 30-50% savings vs. EU/US suppliers for surgical kits, impression copings, and healing components
- Technology Parity: ISO-certified Chinese manufacturers now match EU quality in CBCT (≤5μm resolution), intraoral scanners (sub-20μm accuracy)
- Supply Chain Resilience: Post-2025 geopolitical shifts have strengthened China’s position as primary OEM hub for dental distributors
3-Step Sourcing Protocol for Dental Implant Support Systems
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Critical Risk: 32% of low-cost “implant system” suppliers fail ISO 13485:2024 compliance (2025 DIA Audit Report). Always verify:
| Credential | 2026 Verification Method | Red Flags |
|---|---|---|
| ISO 13485:2024 | Request certificate + factory audit report via SGS/BV. Cross-check IAF database (iaf.nu) | Generic “ISO” claim without 13485:2024 suffix; certificates issued by obscure bodies (e.g., “China Quality Certification”) |
| CE Marking (MDR 2017/745) | Demand EU Authorized Rep letter + NB number verification at ec.europa.eu | CE logo without 4-digit NB number; self-declaration for Class IIb devices |
| US FDA Registration | Confirm facility registration via FDA Establishment Search (DUNS required) | Claims of “FDA Approved” (implants require PMA; China suppliers only register facilities) |
Pro Tip: Require batch-specific CoA (Certificate of Analysis) for surgical-grade titanium (ASTM F136) in abutments.
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers have shifted from rigid MOQs to tiered pricing. Optimize using 2026 market standards:
| Product Category | 2026 Standard MOQ | Cost-Saving Strategy |
|---|---|---|
| Surgical Implant Kits (12-piece) | 50 units | Negotiate 15% discount at 200+ units; share freight costs for LCL shipments |
| Custom Abutments (OEM) | 200 units (per design) | Use “design bank” approach: Pay 1-time mold fee ($850) for 5 shared designs across distributor network |
| CBCT Systems | 3 units | Bundle with chairs/scanners for 8-12% discount; require 18-month warranty minimum |
Warning: Avoid suppliers offering MOQs below industry standards (e.g., 10 surgical kits) – indicates non-compliant subcontracting.
Step 3: Shipping Terms (DDP vs. FOB: 2026 Cost Analysis)
Hidden logistics costs erode 22% of projected savings (2025 Dental Distributor Survey). Choose based on risk profile:
| Term | When to Use | 2026 Cost Components |
|---|---|---|
| FOB Shanghai | Experienced distributors with in-house logistics | • Factory price • Local China freight ($180/kit) • Ocean freight ($3,200/40ft HC) • +12-18% hidden costs (customs clearance, port demurrage, last-mile) |
| DDP Your Clinic | New importers; clinics; urgent orders | • All-inclusive price (quoted) • Guaranteed final cost • Includes 2026 EU VAT (21%), FDA user fees, cargo insurance • 5-7 day delivery post-shipment |
2026 Best Practice: Demand DDP quotes for first 2 orders to benchmark true landed cost before switching to FOB.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental implant support systems, Carejoy mitigates key 2026 sourcing risks:
- Compliance Assurance: Dual ISO 13485:2024 + CE MDR certification (NB 2797) with real-time audit access
- MOQ Flexibility: Tiered pricing from 30 units (surgical kits) with shared OEM design library
- DDP Expertise: All-inclusive DDP quotes to 47 countries with 99.2% on-time delivery (2025 data)
- Product Range: Integrated implant ecosystem: CBCT (Carejoy iMax 3D), surgical kits, healing abutments, and chair-side milling units
Baoshan District, Shanghai, China | Est. 2005
Verified 2026 Capabilities: Factory Direct • 12-Month Warranty • 72-Hour Sample Dispatch • EN ISO 10651-5:2024 Certified Chairs
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 DDP Quote Template + Compliance Dossier (Ref: DENTAL2026)
Final Recommendation
For dental clinics: Source surgical kits/abutments via DDP terms from pre-vetted partners like Carejoy to avoid compliance risks. For distributors: Negotiate tiered MOQs with shared design libraries to achieve 38-45% gross margins in 2026. Always validate claims against IAF/FDA databases – never rely on self-certification.
Disclaimer: This guide covers dental implant support systems. Titanium implant fixtures (Class III) remain primarily EU/US-manufactured. Verify all regulatory claims with national authorities. Data sources: DIA 2025 Market Report, ISO Survey 2025, Carejoy Logistics Dashboard.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing Dental Implant Systems – Key Technical & Service Considerations
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental implant motor system for international use in 2026? | Dental implant motors typically operate on 100–240V AC, 50/60 Hz, making them compatible with global power standards. However, clinics and distributors must confirm the input voltage range specified by the manufacturer. For international deployment, ensure the system includes an IEC 60601-1 certified power supply with over-voltage and surge protection. Dual-voltage models with automatic switching are recommended for multi-region distribution networks to avoid transformer dependency and ensure patient safety compliance. |
| 2. Are spare parts for dental implant motors and handpieces readily available, and what is the typical lead time for critical components? | Reputable manufacturers provide comprehensive spare parts support, including drive couplings, sterilizable handpiece heads, O-rings, and torque-limiting gears. In 2026, leading OEMs offer 7–10 year parts availability guarantees post-discontinuation. Distributors should confirm access to regional spare parts hubs; standard lead times range from 3–7 business days for in-stock items. We recommend maintaining a clinic-level inventory of high-wear components and verifying SLA terms with suppliers to minimize downtime. |
| 3. What does the installation process for a new dental implant system involve, and is on-site technician support required? | Installation includes hardware mounting (motor console, footswitch, handpiece holder), software calibration, and integration with existing dental units or chairside systems. While plug-and-play models are available, on-site technician commissioning is strongly recommended to ensure torque accuracy, software validation, and compliance with ISO 16067-2 standards. Most premium suppliers include one-time professional installation in the purchase agreement, particularly for networked or CAD/CAM-integrated implant platforms. |
| 4. What is the standard warranty coverage for dental implant motors and associated instrumentation in 2026? | As of 2026, the industry standard is a 2-year comprehensive warranty covering defects in materials and workmanship for implant motors and control units. High-torque handpieces typically carry a 1-year warranty. Extended warranties up to 5 years are available through distributors. Note: Warranties are void if maintenance logs are incomplete or non-OEM parts are used. Cybersecurity patches and firmware updates are now included under warranty for smart implant systems. |
| 5. How are software updates and technical support handled under the warranty agreement? | Warranty agreements now include remote diagnostic access and biannual software updates for implant planning integration, torque protocol enhancements, and cybersecurity compliance. Technical support is available 24/7 via secure portal with average response time under 2 hours for critical failures. Distributors receive dedicated support channels and training to assist end-user clinics, ensuring continuity of care and minimizing equipment downtime. |
Need a Quote for How Much Do Dental Implants Costs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


