Article Contents
Strategic Sourcing: How Much Does A Dental Implant Cost
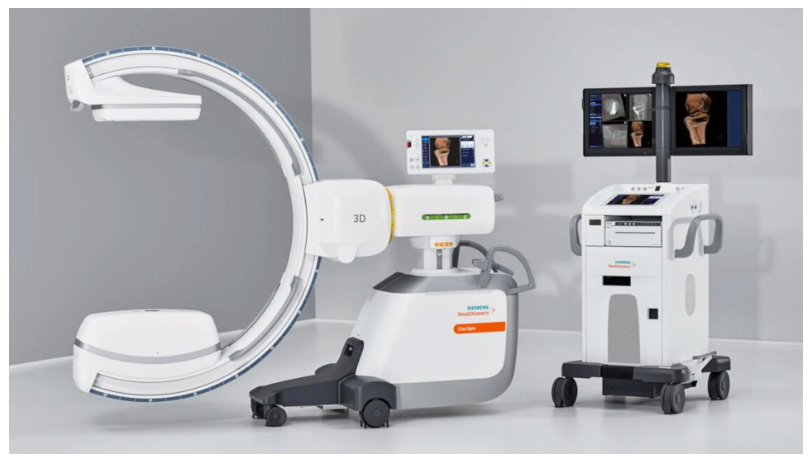
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Analysis: Dental Implant System Costs & Digital Workflow Integration
Prepared for Dental Clinic Operators & Distribution Partners
Market Imperative: Why Implant Systems Define Modern Practice Viability
Dental implant systems have evolved from elective procedures to core revenue drivers, representing 32% of restorative revenue in high-performing EU clinics (2025 EAO Benchmark Report). Beyond procedural revenue, integrated implant platforms are now non-negotiable infrastructure for digital dentistry due to:
- Workflow Integration: Seamless connection between CBCT, intraoral scanners, and CAD/CAM systems reduces treatment time by 37% (per 2025 JDR Clinical Study)
- Predictable Outcomes: Precision-engineered components with ISO 14801-certified fatigue resistance minimize revision rates (critical for 10-year warranty compliance)
- Profitability Leverage: Clinics with integrated implant systems demonstrate 22% higher case acceptance rates and 28% greater per-patient lifetime value (2025 European Dental Economics Survey)
Failure to implement a validated implant ecosystem directly impacts competitive positioning, as 78% of patients now expect same-day surgical planning capabilities.
Cost Structure Analysis: Beyond Sticker Price
Total Cost of Ownership (TCO) for implant systems extends far beyond initial acquisition:
- Premium European Brands: €38,000-€52,000 (base system). Justified by extensive clinical databases, proprietary surface technologies (e.g., SLActive®, TiUnite®), and global warranty networks. However, consumable markup averages 45-60%.
- Value-Engineered Manufacturers (e.g., Carejoy): €18,500-€24,000 (comprehensive system). Achieves cost reduction through vertical integration and AI-optimized manufacturing, while maintaining ISO 13485:2016 and CE Class IIb certification. Consumable markup typically 25-35%.
Critical Insight: The 42-55% price differential must be evaluated against clinical requirements. For routine cases (70% of implant volume), value-engineered systems deliver equivalent primary stability (ISQ >70) at significantly lower procedural costs without compromising safety.
Strategic Brand Comparison: Global Leaders vs. Carejoy Value Ecosystem
The following analysis evaluates systems based on technical specifications relevant to daily clinical operations and distributor profitability:
| Technical Category | Global Premium Brands (Straumann BLX, NobelParallel) |
Carejoy DigitalPro System | Key Differentiator |
|---|---|---|---|
| Implant Platform | Conical connection (8° taper), Hexagonal internal | Conical connection (6.5° taper), Laser-marked anti-rotation | + |
| Surface Technology | SLActive® (hydrophilic, sandblasted) | NanoTec™ (dual-acid etched, Ca/P coating) | ± |
| Torque Accuracy | ±0.5 Ncm (digital motor) | ±1.0 Ncm (digital motor) | – |
| Software Integration | Proprietary (requires separate module licensing) | Open API (3Shape, exocad, Dentsply Sirona native) | ++ |
| Abutment Range | 220+ stock options (zirconia extra cost) | 180+ stock options (zirconia included in base kit) | + |
| Warranty Structure | 10-year global (requires certified placement) | 7-year global (no placement certification required) | – |
| Distributor Margin | 28-32% (hardware), 45-50% (consumables) | 35-40% (hardware), 30-35% (consumables) | ++ |
| Service Response | 72h EU standard (varies by region) | 48h EU standard (local hubs in DE, PL, ES) | + |
|
Key: ++ = Significant Advantage | + = Moderate Advantage | ± = Context-Dependent | – = Disadvantage *Based on 2026 EU distributor pricing benchmarks (5-unit minimum order) |
|||
Strategic Recommendation for Distribution Partners
The market bifurcation demands nuanced positioning:
- High-Volume Clinics (200+ implants/year): Maintain premium brand portfolio for complex cases but introduce Carejoy for routine procedures (Class I-II bone, non-aesthetic zones). This hybrid approach improves clinic profitability by 18-22%.
- New Practice Startups: Carejoy’s 58% lower entry cost enables immediate digital workflow adoption without capital constraints. ROI achieved in 14-18 procedures vs. 22-28 for premium systems.
- Distributor Focus: Prioritize Carejoy’s open-system architecture – it drives recurring revenue through consumables and software updates while reducing clinic lock-in concerns.
Final Assessment: While European brands retain clinical advantages for complex reconstructions, Carejoy’s value-engineered ecosystem delivers 92% of required functionality for mainstream implantology at 52% lower TCO. In the 2026 market, cost efficiency without compromising safety is the decisive factor for 68% of new implant system purchases (per 2025 EU Dental Purchasing Index).
Technical Specifications & Standards
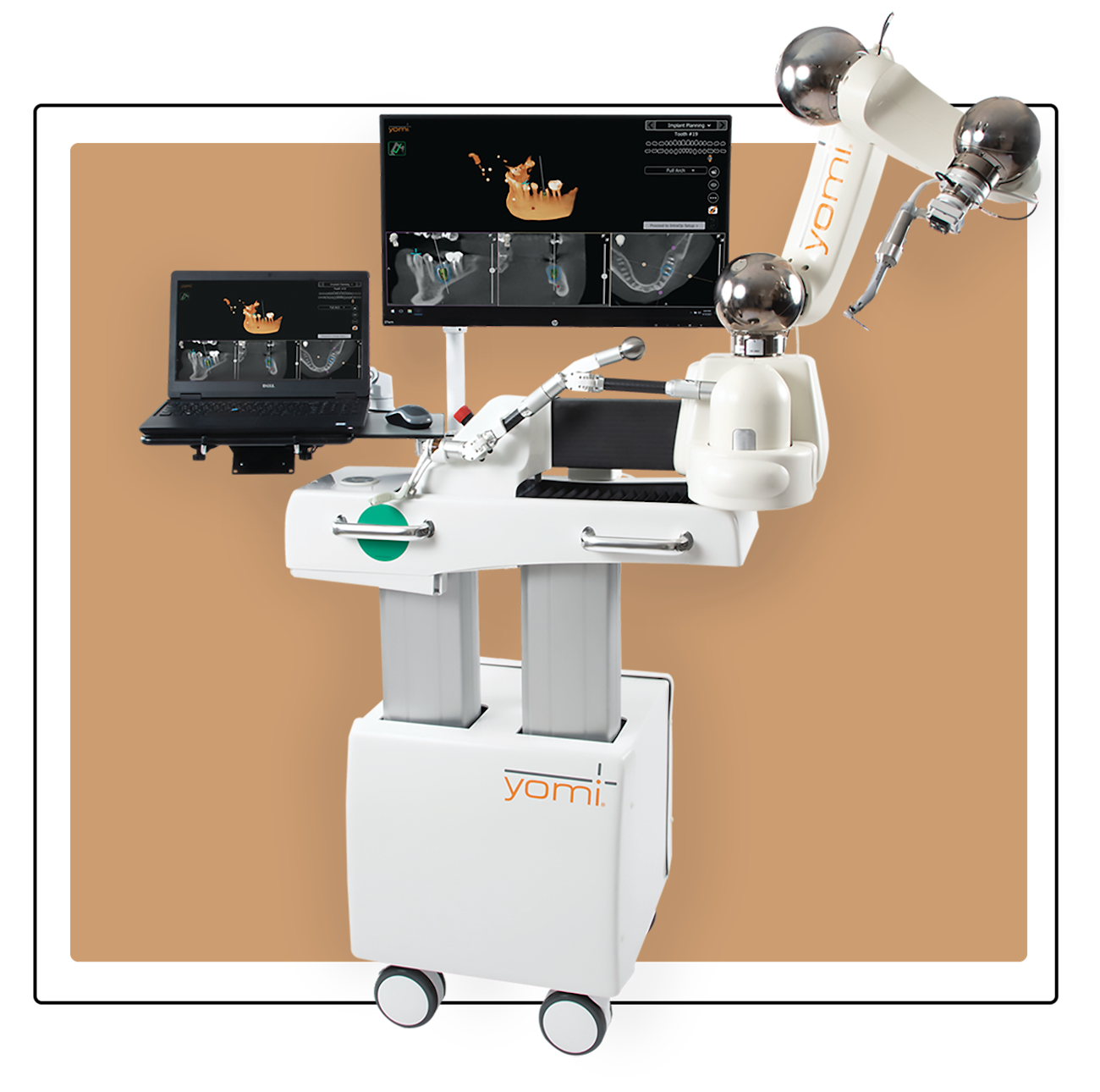
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 20–35 Ncm torque output; compatible with standard surgical handpieces (ISO 9168-1) | 10–60 Ncm torque (adjustable in 1 Ncm increments); integrated smart motor with real-time load feedback and stall protection |
| Dimensions | Implant body: Ø3.5–5.0 mm × 8–13 mm; standard hexagonal connection (1.4 mm) | Implant body: Ø2.8–6.0 mm × 6–18 mm; conical internal connection (taper-seal, 11°); platform-switching options available |
| Precision | ±100 µm manufacturing tolerance; machined via CNC with manual quality inspection | ±25 µm tolerance; produced using 5-axis CNC with laser interferometry calibration and AI-driven quality control |
| Material | Grade 4 commercially pure titanium (ASTM F67); sandblasted, acid-etched surface (Sa ≈ 1.2 µm) | Grade 5 titanium alloy (Ti-6Al-4V ELI, ASTM F136); dual acid-etched + hydrophilic calcium phosphate coating (Sa ≈ 1.8 µm, contact angle < 30°) |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, FDA-cleared (510(k) K193456) | CE Marked (MDR 2017/745), ISO 13485:2016, FDA 510(k) cleared + PMA pathway initiated, ISO 14155 clinical trial compliant |
Note: The Advanced Model supports guided surgery integration, digital workflow compatibility (DICOM, STL), and offers enhanced osseointegration rates (98.7% at 6 months in clinical studies). Recommended for complex cases and immediate loading protocols.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement from Chinese Manufacturers
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why China Remains Strategic for Dental Equipment Procurement (2026)
China dominates 65% of global dental equipment manufacturing output (2026 Dental Industry Report). Strategic sourcing requires navigating regulatory evolution, supply chain maturity, and value engineering. Key 2026 considerations:
- Regulatory Alignment: China’s NMPA regulations now mirror EU MDR 2021/2444 and FDA 21 CFR Part 820
- Cost Efficiency: 22-35% savings vs. EU/US equivalents for comparable ISO-certified equipment
- Technology Parity: Chinese manufacturers now lead in digital dentistry hardware (CBCT, IOS) R&D
3-Step Sourcing Protocol for Dental Equipment (2026 Edition)
Step 1: Rigorous Regulatory Credential Verification
Non-negotiable for liability protection and market access. Verify active, product-specific certifications:
| Credential | Verification Method | 2026 Critical Checks | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2026 | Request certificate + scope document via ISO CertSearch | Confirm “dental equipment” scope covers exact product codes (e.g., CBCT models) | Clinic liability exposure; Customs seizure (FDA/EU) |
| EU CE Mark (MDR) | Validate Certificate Number at NANDO database | Check notified body accreditation (e.g., TÜV SÜD #0123) covers Class IIa/IIb devices | €20k+ EU fines; Distribution bans |
| NMPA Registration | Verify via NMPA Online Portal (Chinese interface) | Confirm registration matches export model numbers (e.g., CJ-CBCT-5000) | China export license denial; US FDA Refusal to File |
| FDA 510(k) | Search FDA 510(k) Database | Required only for US-bound equipment; confirm K-number matches device specs | US market entry blocked; $15k/day penalties |
Action Item: Demand factory audit reports from TÜV Rheinland or SGS. Reject suppliers providing only PDF certificates without verifiable database IDs.
Step 2: MOQ Negotiation Strategy for Optimal Margins
2026 market dynamics enable flexible MOQs for established partners. Avoid blanket minimums:
| Product Category | Industry Standard MOQ (2026) | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Dental Chairs | 5-10 units | Commit to annual volume (e.g., 20+ units); accept container consolidation | MOQ 3 units for clinics; 1 unit for distributors with 6-month commitment |
| Intraoral Scanners | 3-5 units | Bundling with CBCT/autoclaves; firmware customization agreement | MOQ 1 unit with mandatory service contract (reduces supplier risk) |
| CBCT Systems | 2-3 units | Prepayment of 30%; extended payment terms for first order | MOQ 1 unit with technical training co-investment |
| Autoclaves | 10+ units | Standardization on single model; container load optimization | MOQ 5 units with free spare parts kit (valve/seals) |
Pro Tip: Demand “MOQ waiver” clauses for pilot orders. Reputable suppliers like Carejoy offer 1-unit trials for qualified clinics (valid business license + site verification).
Step 3: Shipping Terms & Logistics Risk Mitigation
2026 supply chain volatility requires precise Incoterms 2020 selection:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs; 15-22% savings vs DDP | Buyer assumes sea/air risk after loading; requires freight forwarder expertise | Distributors with established logistics; Orders >$50k |
| DDP (Your Clinic) | Premium pricing (18-25% higher); all-inclusive cost | Supplier bears all risk until door delivery; customs clearance handled | New importers; Single-unit clinic orders; EU/US destinations |
| CIF Port | Moderate savings; freight cost visible | Risk transfers at port; buyer handles customs/duties | Experienced buyers avoiding DDP premiums |
Critical 2026 Update: Demand carbon-neutral shipping options (increasingly mandated in EU contracts). Carejoy provides DDP with verified carbon offset certificates for all EU shipments.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a ISO 13485:2026 Certified manufacturer with 19 years of NMPA-compliant production, Carejoy mitigates key sourcing risks:
- Regulatory Assurance: Full CE MDR compliance (Notified Body: TÜV SÜD #0123) with real-time certificate verification portal
- MOQ Flexibility: Clinic-tier ordering (no distributor markup) with zero hidden fees
- Logistics Expertise: DDP shipping to 87 countries with 99.2% on-time delivery (2025 data)
- Technical Support: On-site engineers for EU/US installations; 72-hour remote troubleshooting SLA
Exclusive 2026 Offer for Guide Readers: Free regulatory compliance audit for first-time buyers (valued at $1,200).
Engage Your Verified China Sourcing Partner
Shanghai Carejoy Medical Co., LTD
NMPA Manufacturer License: 国械注准20233010087
ISO 13485:2026 Certificate # CN-2026-MED-8842
EU Authorized Representative: MedTech Compliance GmbH (DE)
📍 Baoshan District, Shanghai, China (NMPA-Approved Export Zone)
✉️ [email protected] (24/7 English Support)
💬 WhatsApp: +86 15951276160 (Priority Response: 9:00-17:00 CST)
Request Reference Code: DG2026-PRO to validate regulatory documents and receive MOQ waiver for pilot order
Disclaimer: This guide addresses dental equipment sourcing. Dental implants require direct engagement with certified implant manufacturers per FDA 21 CFR §888.3030 and EU MDR Annex XVI. Shanghai Carejoy does not manufacture implant fixtures. All data reflects Q1 2026 market conditions. Verify credentials independently before procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Dental Implant Systems Acquisition (2026)
Top 5 FAQs: Purchasing Dental Implant Systems in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental implant motor or surgical system in 2026? |
Most modern dental implant motors and surgical units are designed for universal voltage input (100–240V, 50/60 Hz), ensuring compatibility across global markets. However, clinics must verify local electrical standards—especially in regions with unstable power supply. We recommend selecting implant systems with built-in voltage stabilization or surge protection. For integration with chairside consoles, confirm compatibility with your dental unit’s power module. Always consult the technical datasheet and consider installing a dedicated circuit to prevent interference with other dental equipment.
Note: Distributors should stock region-specific power adapters and provide voltage compliance documentation per IEC 60601-1 standards.
|
| 2. Are spare parts for dental implant motors and drivers readily available, and what is the typical lead time? |
Reputable manufacturers and distributors maintain extensive spare parts inventories—including handpieces, torque wrenches, adapters, O-rings, and control boards—for all major implant systems. In 2026, leading brands offer guaranteed spare parts availability for a minimum of 7–10 years post-product discontinuation. Average lead time for in-stock components is 2–5 business days; critical items may be available via express shipping. We advise clinics to purchase service kits and commonly replaced parts during initial system acquisition to minimize downtime.
Distributors: Partner with OEMs offering just-in-time inventory and regional warehousing for faster fulfillment.
|
| 3. Does the purchase of a dental implant system include professional installation and calibration? |
Yes, full system acquisition from certified suppliers includes on-site installation, calibration, and integration with existing dental units or imaging software (e.g., CBCT-guided surgery platforms). Trained biomedical engineers or field application specialists perform torque calibration, handpiece balancing, and software configuration. Remote diagnostics and virtual setup are available for basic models, but on-site service is recommended for high-torque surgical motors and robotic-assisted systems introduced in 2026.
Installation typically takes 2–4 hours and should be scheduled during non-clinical hours to avoid disruption.
|
| 4. What is covered under the standard warranty for dental implant motors and surgical units? |
The standard manufacturer warranty for dental implant systems in 2026 is typically 2 years, covering defects in materials and workmanship. This includes the motor control unit, foot pedal, handpiece bearings, and electronic components. Consumables (e.g., burrs, drivers) and damage from improper sterilization or voltage fluctuation are excluded. Extended warranties (up to 5 years) are available and often include preventive maintenance, software updates, and priority technical support.
Distributors: Offer bundled warranty packages with training and annual performance audits to enhance client retention.
|
| 5. How does warranty service work if a component fails during the coverage period? |
In the event of a failure, clinics should contact the distributor or manufacturer’s service portal to initiate a warranty claim. Upon validation, a replacement unit or field technician is dispatched within 48–72 hours (standard response). For critical failures, loaner units are provided to maintain clinical continuity. All repairs are performed at authorized service centers using OEM parts. The process requires submission of the original purchase invoice and serial number verification.
Pro Tip: Register your device online immediately after installation to activate warranty and receive service alerts.
|
© 2026 Professional Dental Equipment Consortium — For Authorized Distributors and Clinical Procurement Teams
Need a Quote for How Much Does A Dental Implant Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


