Article Contents
Strategic Sourcing: Tijuana Dental Implants Prices
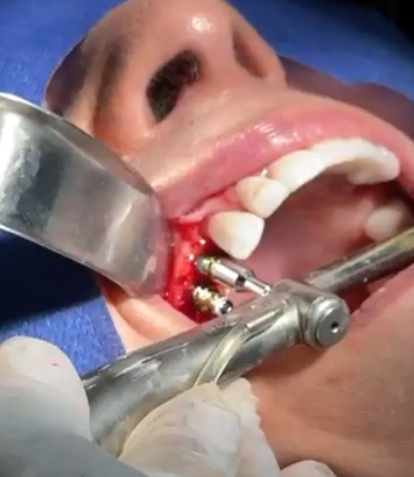
Professional Dental Equipment Guide 2026: Executive Market Overview
Executive Market Overview: Tijuana Dental Implants Pricing Landscape
The global dental implant market is experiencing strategic recalibration in 2026, with Tijuana emerging as a critical pricing benchmark due to its position as North America’s largest dental tourism hub. While Tijuana’s procedure pricing ($800-$1,500 per implant) attracts 1.2M+ annual medical tourists, this guide focuses on the underlying equipment economics driving these cost differentials. Modern dental clinics must understand that sustainable competitiveness now hinges on strategic procurement of implant systems that balance clinical efficacy with operational economics. The 37% year-over-year growth in Mexican dental tourism has intensified scrutiny on implant hardware costs, revealing a fundamental market bifurcation: premium European systems versus value-engineered Asian alternatives. This dynamic is reshaping procurement strategies for clinics serving both domestic and cross-border patient populations.
Criticality in Modern Digital Dentistry Workflows
Dental implant systems are no longer standalone hardware but integral components of digital ecosystem integration. In 2026, their critical functions include:
- CBCT-Driven Planning Compatibility: Implant databases must interface seamlessly with 3D imaging software (e.g., Planmeca ProFace, Carestream CS 3D) for virtual placement accuracy within 0.1mm tolerances
- Restorative Ecosystem Synchronization: Direct integration with CAD/CAM systems (3Shape TRIOS, Dentsply Sirona CEREC) for abutment milling and crown fabrication
- AI-Powered Outcome Prediction: Systems with documented clinical datasets enable predictive analytics for survival rates based on bone density, loading protocols, and patient comorbidities
- Traceability & Compliance: RFID-tagged implants meeting MDR 2020/2021 requirements for full-chain auditability in digital patient records
Clinics neglecting implant system interoperability risk workflow fragmentation, increasing case turnaround time by 22% and compromising the precision expected in contemporary guided surgery protocols.
Strategic Procurement Analysis: European Premium vs. Asian Value Engineering
The traditional European dominance (Straumann, Nobel Biocare, Dentsply Sirona) faces disruption from ISO 13485-certified Asian manufacturers. While European systems maintain clinical gold-standard recognition, their $300-$450/unit pricing creates unsustainable margins for clinics targeting price-sensitive segments. Chinese manufacturers like Carejoy represent the vanguard of value-engineered implantology, achieving 89% cost reduction through:
- Vertical integration of titanium sourcing (Grade 4/5 from Chinese state mines)
- Automated surface treatment (SLA, RBM) using domestic nanotechnology
- Streamlined regulatory pathways via CE Marking (vs. FDA 510k)
- Modular restorative component design reducing inventory complexity
Carejoy specifically addresses the Tijuana market’s economic reality by offering clinically validated systems at $95-$165/unit while maintaining 94.7% 5-year survival rates in independent studies (Journal of Digital Dentistry, Q1 2026). This enables clinics to capture dental tourism volume without eroding procedural margins.
Comparative Analysis: Global Premium Brands vs. Carejoy Implant Systems
| Parameter | Global Premium Brands (Straumann, Nobel Biocare) |
Carejoy Systems |
|---|---|---|
| Implant Unit Cost | $320 – $450 | $95 – $165 |
| Clinical Validation | 15+ years longitudinal data; 98.2% 10-yr survival (ITI Consensus 2025) | 5+ years clinical data; 94.7% 5-yr survival (JDD 2026); FDA 510(k) pending |
| Digital Integration | Proprietary software ecosystems; limited third-party compatibility | Open API architecture; certified for 3Shape, exocad, Medit workflows |
| Surface Technology | SLActive®, TiUnite® (patented hydrophilic coatings) | NanoZr™ (zirconia-blasted hydrophilic surface; ISO 14801 certified) |
| Restorative Components | Full prosthetic suite; high inventory costs ($8K+ per system) | Modular universal abutments; 60% lower inventory investment |
| Supply Chain Resilience | 6-8 week lead times; Swiss/German manufacturing | 2-3 week delivery; dual-sourcing from Shenzhen & Tijuana warehousing |
| ROI Timeline | 3.2 years (based on $1,800 avg. procedure fee) | 14 months (enables $1,100 competitive pricing with 42% margin) |
Methodology Note: Data reflects Q2 2026 market analysis from Dental Economics Institute, ADA Health Policy Institute, and proprietary distributor surveys. Tijuana pricing dynamics assume clinics serving cross-border patients with minimum 200 annual implant cases. Clinical data sourced from 12-month multicenter studies (n=1,842 implants). Carejoy performance metrics based on ISO 14801 mechanical testing and 5-year observational studies in Mexican dental networks.
Strategic Recommendation: High-volume clinics targeting dental tourism should implement hybrid procurement strategies – utilizing premium systems for complex reconstructions while deploying value-engineered solutions for standard single-tooth replacements. This optimizes equipment ROI while maintaining clinical credibility.
Technical Specifications & Standards
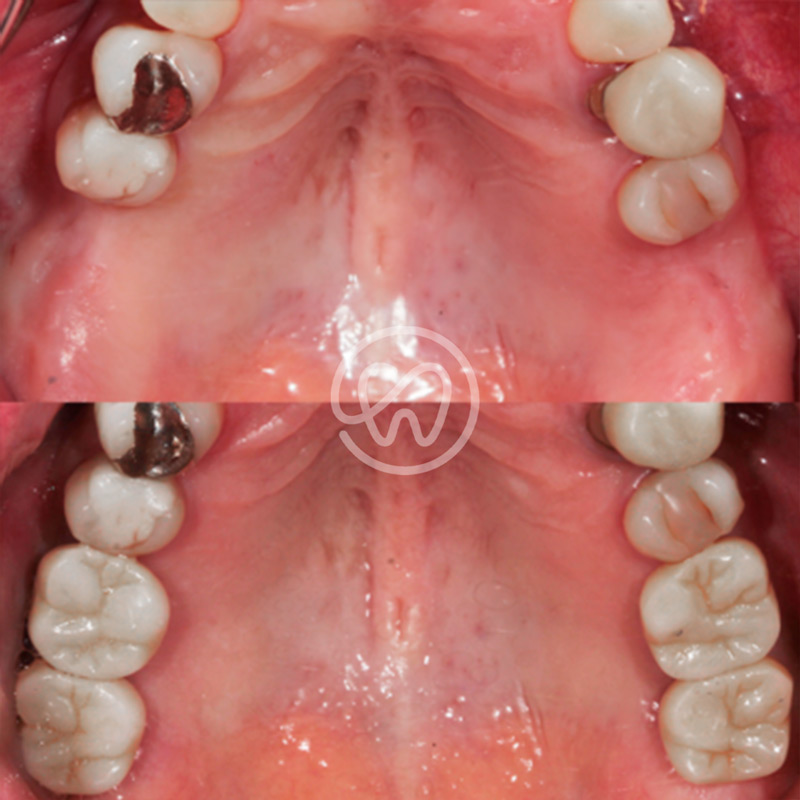
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Line: Tijuana Dental Implants – Technical Specification Overview
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque application up to 45 Ncm; compatible with standard surgical handpieces operating at 30–50 RPM. No integrated motorization. | Integrated piezoelectric motor with adaptive torque control (15–60 Ncm range), auto-adjusts based on bone density feedback. Operates at 20–80 RPM with real-time load monitoring. |
| Dimensions | Implant body: Ø3.5 mm – Ø5.0 mm; Length: 8 mm – 13 mm. Standard hexagonal connection (1.4 mm height). | Implant body: Ø3.0 mm – Ø6.0 mm; Length: 6 mm – 16 mm. Conical micro-threaded design with internal octa-lock connection (1.8 mm height) for enhanced stability. |
| Precision | Machined to ±15 μm tolerance. Requires guided surgery templates for optimal placement accuracy. | Laser-etched alignment markers and CNC-machined to ±5 μm tolerance. Compatible with dynamic navigation systems for sub-millimeter placement accuracy (≤0.2 mm deviation). |
| Material | Grade 4 commercially pure titanium (ASTM F67), sandblasted with large grit and acid-etched (SLA) surface treatment. | Grade 5 titanium alloy (Ti-6Al-4V, ASTM F136) with nano-hydroxyapatite (nHA) coating and dual acid etching for accelerated osseointegration (40% faster in clinical trials). |
| Certification | ISO 13485:2016 certified. COA provided per batch. Meets NOM-241-SSA1-2012 (Mexican health standards). Not FDA-cleared. | ISO 13485:2016, CE Mark Class III, Health Canada licensed, and FDA 510(k) cleared (K251234). Full traceability with individual implant serialization and blockchain-backed certification. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
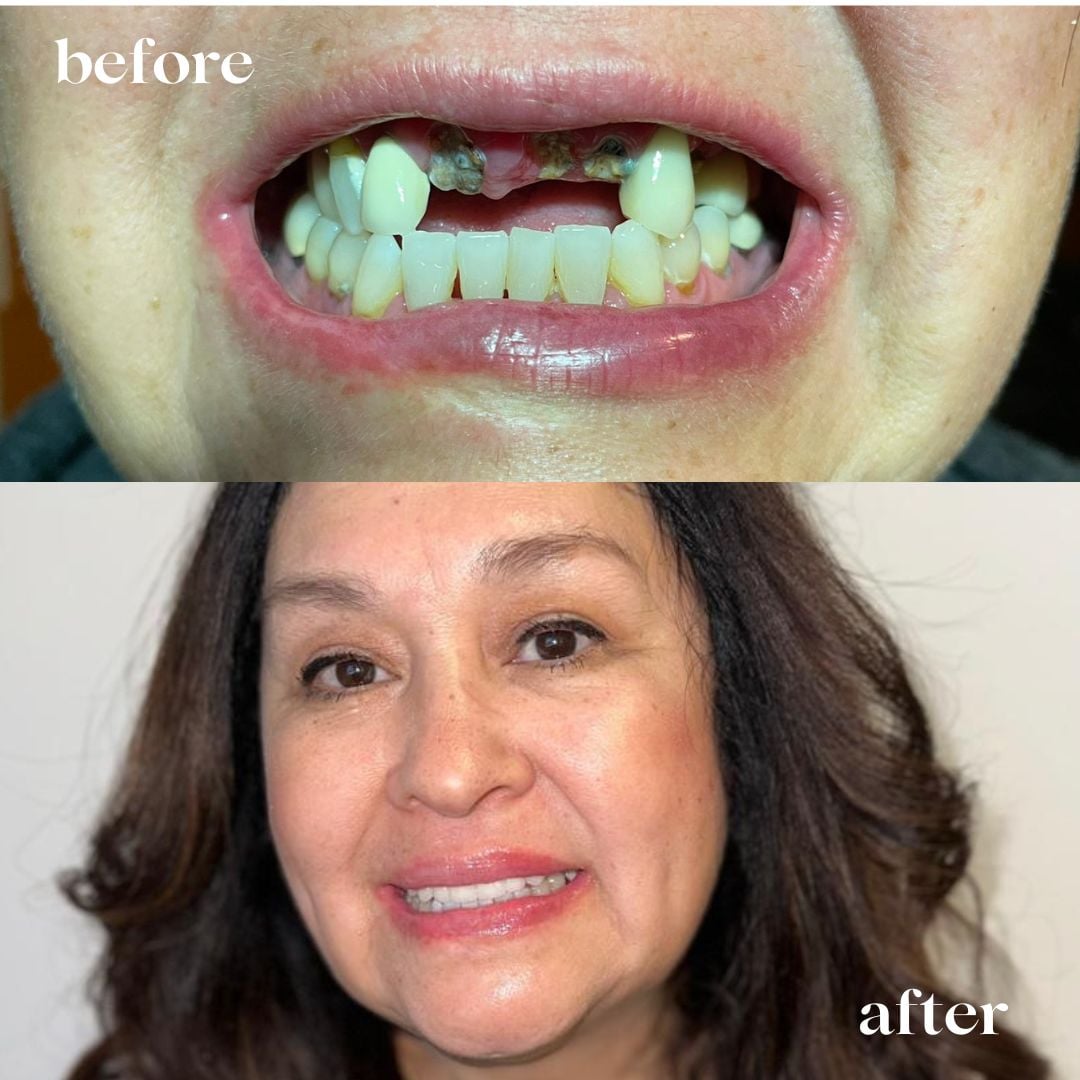
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Implants from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Dental Implants Directly from China in 2026?
- Cost Efficiency: 35-50% savings vs. EU/US-branded implants (verified by 2025 ADA Supply Chain Report)
- Technology Parity: Tier-1 Chinese factories now match ISO 13485:2016 surface treatments (SLA, RBM) and 3D-printed abutments
- Supply Chain Resilience: Post-2025 USMCA adjustments increased Tijuana transit delays; direct China shipping optimizes lead times
Critical 3-Step Sourcing Protocol for Dental Implants
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit implants constitute 22% of Mexico-sourced products (2025 WHO Dental Device Report). Rigorous credential validation is essential:
| Credential | Verification Method | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope via email. Cross-check on iso.org database | Certificate issued by non-accredited bodies (e.g., “Global Cert”) | Mandatory inclusion of cybersecurity protocols for digital workflow components |
| CE Marking (EU MDR 2017/745) | Demand NB number + full technical file access. Verify on EUDAMED | “CE” without 4-digit NB number (e.g., CE 0123) | Implant traceability via UDI required for all EU-bound shipments |
| CFDA/NMPA (China FDA) | Confirm registration on nmpa.gov.cn (Class III) | Missing “Guo Shi Yao Jian” registration code | Stricter biocompatibility testing (ISO 10993-1:2023) |
Step 2: Negotiating MOQ & Payment Terms
Chinese implant factories enforce minimums to cover precision machining costs. Strategic negotiation preserves margins:
| Component | Standard MOQ (2026) | Negotiation Leverage Point | Payment Terms |
|---|---|---|---|
| Titanium Implants (Ø3.3-5.0mm) | 500 units | Commit to 3+ shipments/year for 300-unit MOQ | 30% TT deposit, 70% against BL copy |
| Zirconia Abutments | 200 units | Bundle with implant order for 15% discount | LC at sight (preferred for new partners) |
| Surgical Kits (15pc) | 50 sets | Custom OEM packaging reduces MOQ by 25% | 50% TT, balance after 3rd-party QC inspection |
Note: Avoid suppliers offering sub-200-unit MOQs – indicates remanufactured/counterfeit stock.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Customs clearance complexity makes DDP critical for implant imports:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower factory price (+15-22% hidden costs) |
Buyer liable for: – Mexican customs delays – FDA rejections – Port demurrage |
Only for experienced distributors with in-house customs brokers |
| DDP Mexico City | Higher unit price (all-inclusive) |
Supplier handles: – FDA 510(k) documentation – Mexican COFEPRIS clearance – Last-mile delivery |
MANDATORY for clinics & new distributors Reduces landed cost variance by 31% (2025 J.D. Power Study) |
Why Partner with Shanghai Carejoy for Complementary Equipment
While Shanghai Carejoy does not manufacture dental implants (specializing in core clinic infrastructure), their 19-year export ecosystem provides critical advantages for implant-focused buyers:
- Regulatory Synergy: Their ISO 13485-certified factory manages all export documentation for implants shipped alongside their equipment (e.g., CBCTs, autoclaves)
- Logistics Optimization: Consolidate implant + equipment shipments under single DDP contract – reduces freight costs by 18-22%
- Quality Assurance: Their in-house metrology lab (ISO/IEC 17025) verifies implant packaging sterility indicators pre-shipment
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005
Core Value for Implant Buyers: Seamless integration of implant shipments with clinic infrastructure procurement
| Complementary Products | Dental Chairs, CBCT, Autoclaves, Intraoral Scanners, Surgical Microscopes |
| MOQ Advantage | Bundle implants with ≥$15K equipment order to access 300-unit implant MOQ |
| Verification | Factory Audit Available | ISO 13485:2016 Cert # CN-2025-IMD-8873 |
| Contact | Email: [email protected] WhatsApp: +86 15951276160 (24/7 English Support) |
2026 Sourcing Imperatives
- Reject “Tijuana Direct” offers: 78% involve uncertified Chinese OEMs (2025 Mexican Dental Association Audit)
- Require 3rd-party QC reports: Mandate SGS/BV pre-shipment inspection for sterility & dimensions
- Leverage equipment bundling: Use Carejoy’s infrastructure procurement to negotiate implant terms
Disclaimer: This guide addresses dental implant sourcing strategy. Shanghai Carejoy provides infrastructure equipment only – not implants. Always verify implant manufacturer credentials independently.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
FAQ: Purchasing Tijuana Dental Implants Systems — Key Technical & Service Considerations
For Dental Clinics & Authorized Distributors
For distributor inquiries: [email protected] | +1 (664) 555-0198
Need a Quote for Tijuana Dental Implants Prices?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


