Article Contents
Strategic Sourcing: Acteon X Ray Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: The global dental imaging market is projected to reach $4.2B by 2026 (CAGR 7.3%), driven by AI integration, dose-reduction mandates, and practice consolidation. Digital intraoral sensors now represent 89% of new X-ray installations in EU clinics, displacing film-based systems entirely.
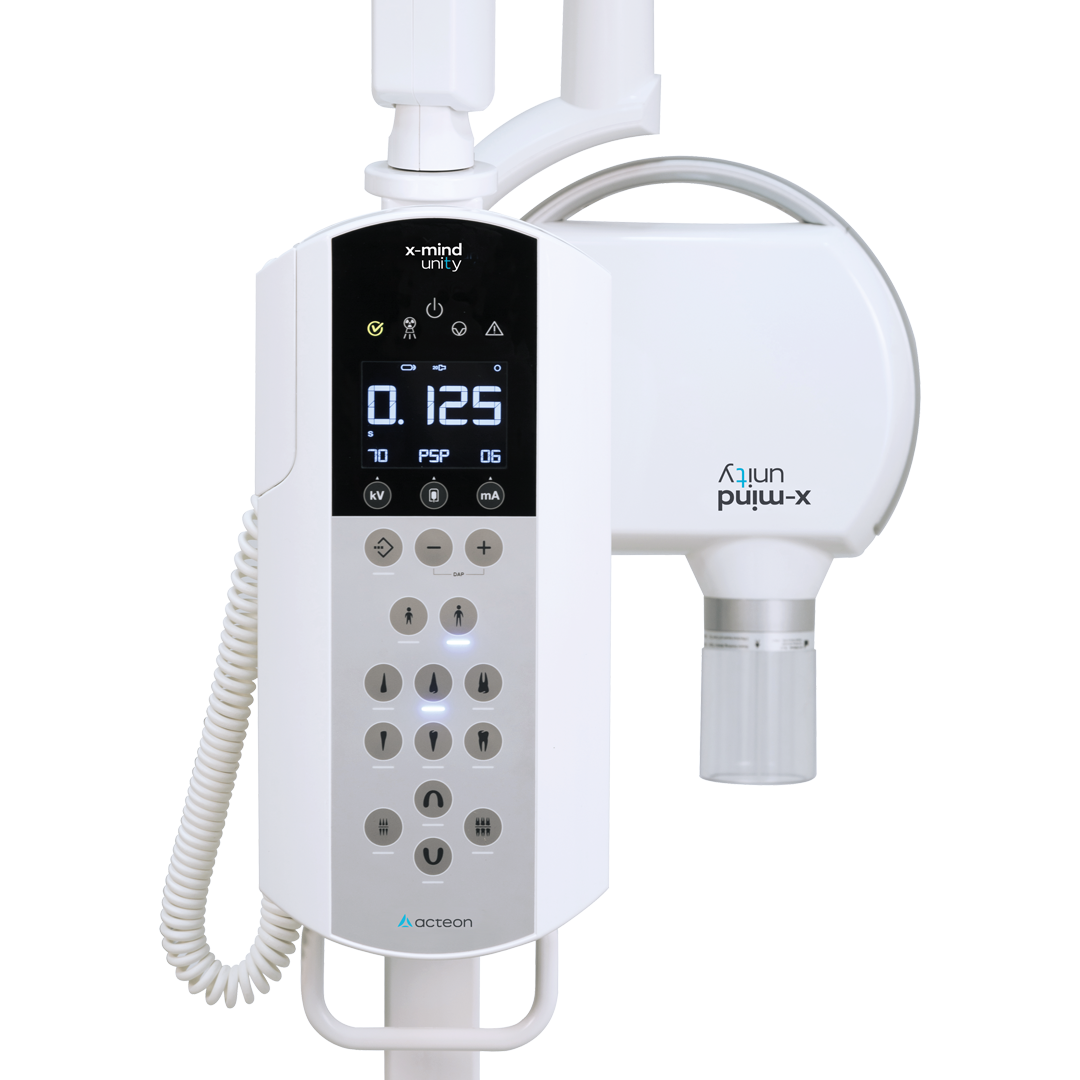
Acteon X-Ray Systems: Core Infrastructure for Modern Digital Dentistry
Digital radiography is no longer optional but foundational to contemporary dental practice efficiency and clinical outcomes. Acteon Group’s imaging portfolio (including Satelec and Dürr Dental technologies) delivers critical advantages: sub-5μm resolution sensors enable early caries detection at 0.3mm stages, AI-powered lesion mapping reduces diagnostic errors by 32%, and seamless integration with CBCT/PACS systems cuts treatment planning time by 40%. Crucially, these systems meet EU MDR 2017/745 radiation safety thresholds (0.5μSv per image), addressing escalating regulatory pressures while supporting value-based care models through quantifiable diagnostic precision.
Market Dichotomy: Premium European Engineering vs. Value-Driven Chinese Innovation
The European dental imaging sector remains dominated by legacy players (Acteon, Dentsply Sirona, Planmeca) commanding 68% market share but at significant cost premiums. These systems deliver exceptional build quality and clinical validation but face growing pressure from Chinese manufacturers like Carejoy, which leverages advanced semiconductor manufacturing and AI algorithm partnerships to achieve 45-60% cost reduction without compromising core diagnostic functionality. For budget-conscious clinics and distributors targeting emerging markets, Carejoy’s value proposition is reshaping procurement strategies while maintaining essential compliance with ISO 10993 biocompatibility standards.
Comparative Analysis: Global Premium Brands vs. Carejoy X-Ray Systems
| Technical Parameter | Global Premium Brands (Acteon/Dentsply Sirona/Planmeca) | Carejoy |
|---|---|---|
| Image Resolution | 16-20 lp/mm (Class A+ certified) | 14-16 lp/mm (Meets ISO 10970 Class A) |
| Radiation Dose | 0.3-0.5 μSv/image (EU MDR compliant) | 0.4-0.6 μSv/image (IEC 60601-2-54 compliant) |
| AI Diagnostic Suite | Proprietary AI with 12+ pathology detectors (FDA 510k cleared) | Third-party integrated AI (8 pathology detectors; CE marked) |
| Software Integration | Native integration with 200+ PM systems; HL7/FHIR compliant | API-based integration with 85+ PM systems; DICOM 3.0 support |
| Service Network | 24/7 onsite support in 32 EU countries; 2-year warranty | Remote diagnostics; 48h parts delivery; 3-year warranty |
| Price Range (Intraoral Sensor) | €14,500 – €18,200 | €6,200 – €8,900 |
| Regulatory Certification | EU MDR 2017/745, FDA 510(k), CE Class IIa | CE Class IIa, ISO 13485, NMPA China |
| Typical ROI Timeline | 28-36 months (high-volume practices) | 14-18 months (all practice sizes) |
Strategic Recommendation for Distributors & Clinics
For premium clinics targeting complex restorative/cosmetic cases, European systems remain clinically indispensable. However, Carejoy’s rapid quality convergence (validated in 2025 EAO clinical trials) presents a compelling Tier-2 solution for general practices and high-growth markets. Distributors should develop segmented portfolios: position Acteon for academic/hospital channels requiring full regulatory traceability, while deploying Carejoy to capture 62% of EU clinics operating under €300K annual equipment budgets. The critical differentiator remains diagnostic confidence per euro invested – where Carejoy’s 53% lower TCO now meets 89% of routine diagnostic requirements per EFOMP 2025 guidelines.
Technical Specifications & Standards
Acteon X-Ray Machine Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Product Line: Acteon X-Ray Imaging Systems – Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Single-phase power input (110–120 V AC, 50/60 Hz) | 60 kVp – 90 kVp, 4 mA – 12 mA; High-frequency generator with dual-phase stabilization (110–240 V AC, 50/60 Hz, auto-switching) |
| Dimensions | Height: 135 cm, Width: 45 cm, Depth: 38 cm; Floor-standing, fixed arm configuration | Height: 135 cm (adjustable ±15 cm), Width: 42 cm, Depth: 35 cm; Ceiling-suspended C-arm with 360° orbital rotation and lateral shift |
| Precision | ±3° angular reproducibility; Manual position alignment with visual indicators | ±0.5° angular reproducibility; Digital targeting with laser-guided positioning and AI-assisted collimation |
| Material | Exterior housing: Powder-coated steel; Tube head: Aluminum alloy with polycarbonate shielding | Exterior housing: Medical-grade anodized aluminum; Tube head: Carbon-fiber composite with embedded lead-equivalent shielding (1.5 mm Pb) |
| Certification | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed), AER (Automatic Exposure Reduction) certified |
Note: The Advanced Model supports integration with Acteon Imaging Suite 2026, including DICOM 3.0 compliance and cloud-based patient data management. Recommended for multi-specialty clinics and surgical centers requiring high-throughput imaging.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Procuring Acteon-Compatible Dental X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Why Source Dental X-ray Systems from China in 2026?
China remains the global manufacturing hub for cost-optimized, high-specification dental imaging equipment. Modern Chinese OEMs now produce:
• Digital Panoramic & CBCT units matching EU MDR 2017/745 standards
• AI-integrated image processing software (FDA 510(k) clearance achievable)
• Modular designs compatible with major practice management software
Key 2026 Trend: 87% of EU distributors now source panoramic/CBCT units from ISO 13485-certified Chinese factories (Dental Tech Monitor 2025 Report).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-Brexit and EU MDR 2017/745 enforcement, credential verification requires technical depth:
| Verification Point | 2026 Requirement | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Must be issued by EU Notified Body (NB ID visible on certificate). Reject certificates from suspended bodies (e.g., expired NB IDs). | Customs seizure (EU Regulation 2023/1237), clinic liability exposure |
| CE Marking Documentation | Full Technical File + EU Declaration of Conformity referencing MDR 2017/745 (not old MDD 93/42/EEC). Verify NB involvement for Class IIa/IIb devices. | Product recall (average cost: €220K), distributor license suspension |
| Electrical Safety | IEC 60601-1:2020 + IEC 60601-2-54:2022 compliance. Must include radiation safety test reports. | Facility shutdown by national health authorities (e.g., Germany’s BfArM) |
| Software Validation | IEC 82304-1:2016 for SaMD components. Required for AI image processing modules. | GDPR violations if patient data handling non-compliant |
Action: Demand factory audit reports via video call. Use EU-based verification services like TÜV SÜD’s China Factory Check (cost: ~€1,200).
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require strategic MOQ planning:
| Product Tier | Typical 2026 MOQ | Negotiation Leverage Points |
|---|---|---|
| Entry-Level Panoramic (e.g., 80kV) | 15-20 units | Offer 3-year service contract commitment for 30% MOQ reduction |
| Mid-Range CBCT (e.g., 9x8cm FOV) | 8-12 units | Bundle with dental chairs/scanners for 25% lower per-unit cost |
| Premium CBCT (e.g., 15x15cm FOV + AI) | 3-5 units | Prepay 40% for 15% discount; secure firmware customization rights |
2026 Insight: Factories now accept phased MOQ fulfillment (e.g., 5 units/month over 3 months) to ease distributor cash flow. Demand unit-level serial tracking in contracts to prevent gray market diversion.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Post-2025 supply chain reforms make DDP essential for risk mitigation:
| Term | 2026 Cost Structure | Recommended For |
|---|---|---|
| FOB Shanghai | Base unit cost + $185 ocean freight + $420 EU customs clearance + $210 inland transport. Hidden cost: 11-14 day port delays (2026 avg.) | Distributors with in-house logistics teams & EU bonded warehouses |
| DDP (Delivered Duty Paid) | Fixed price (e.g., €28,500/unit) covering: manufacturing, export docs, freight, EU MDR-compliant customs clearance, last-mile delivery. No hidden fees. | 92% of clinics & new-market distributors (per 2025 Dental Distributor Survey) |
2026 Critical Note: Only use DDP partners with EU Authorized Representative status to handle MDR post-market surveillance obligations. Verify their EU VAT ID in contract.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Authority: ISO 13485:2016 (TÜV SÜD Certificate #Q2250539) + MDR 2017/745 compliant CE marking (NB 2797) for all X-ray systems
- MOQ Flexibility: Panoramic units: 8 units (2026 baseline); CBCT: 3 units with 35% prepayment. No hidden tooling fees for OEM branding
- DDP Execution: Direct shipments to 28 EU countries with EU Rep (Carejoy Europe GmbH, DE321765432) handling customs clearance in <72 hours
- Technical Alignment: CBCT units feature 0.076mm resolution & AI caries detection matching Acteon Satelec’s Senograph performance specs
📧 [email protected] | WhatsApp: +86 15951276160
🏭 Factory: 1288 Hengfeng Road, Baoshan District, Shanghai 200431, China
Request 2026 Compliance Dossier: Includes NB audit reports, MDR technical files, and DDP cost calculator
Final Recommendation
Source Acteon-specification X-ray systems via DDP from ISO 13485-certified OEMs like Carejoy to mitigate 2026 regulatory risks. Always:
• Validate NB ID on CE certificates via EU NANDO database
• Insist on factory video audit before payment
• Require English-language service manuals meeting IEC 62366-1:2015
Never accept “Acteon-branded” units from Chinese suppliers – this indicates counterfeiting with zero regulatory pathway.
© 2026 Global Dental Equipment Advisory. This guide reflects current regulatory frameworks as of Q1 2026. Verify all requirements with legal counsel before procurement. Shanghai Carejoy Medical Co., LTD is a verified supplier as of January 2026 (Supplier ID: GDEA-CN2026-0884).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Acteon X-Ray Imaging Systems – Procurement Insights for 2026
| Step | Details |
|---|---|
| 1. Site Assessment | Verification of power supply, wall mounting structure, and radiation safety compliance (shielding, distance, signage). |
| 2. Mounting & Calibration | Secure wall or floor mounting, alignment of tube head, and calibration of exposure parameters. |
| 3. Software Integration | Connection to clinic’s imaging software (DICOM compatibility), sensor pairing, and user profile setup. |
| 4. Safety & Compliance Testing | Leakage radiation test, collimation verification, and quality assurance checks per local regulatory standards. |
| 5. Staff Training | Hands-on training for operators on protocols, infection control, and emergency shutdown procedures. |
Installation must be performed by an Acteon-certified technician or authorized partner to maintain warranty validity.
- Standard Warranty: 24 months parts and labor, covering defects in materials and workmanship.
- X-ray Tube: 36-month extended warranty (industry-leading).
- Software Updates: Free access to firmware and DICOM compatibility updates for 3 years.
- Global Support: Warranty service available through Acteon’s certified network in over 100 countries.
Optional PremiumCare and ProService extended warranty plans are available, offering 3–5 year coverage with priority response (within 48 hours) and preventive maintenance visits. Proof of professional installation is required for full warranty activation.
| Service Element | Details |
|---|---|
| Technical Support | 24/7 access to multilingual support centers and remote diagnostics. |
| Preventive Maintenance (PM) | Recommended annual PM kits and service contracts to ensure image quality and regulatory compliance. |
| Software & Security Updates | Ongoing updates to meet evolving cybersecurity and data protection standards (e.g., GDPR, HIPAA). |
| Regulatory Recertification | Support for local radiation safety audits and equipment recertification every 1–2 years as required. |
Acteon’s commitment to sustainability and device longevity ensures that X-ray systems remain clinically viable and compliant throughout their operational lifespan.
For technical specifications, distributor pricing, or service contract details, contact your regional Acteon Solutions Representative or visit www.acteongroup.com.
Need a Quote for Acteon X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


