Article Contents
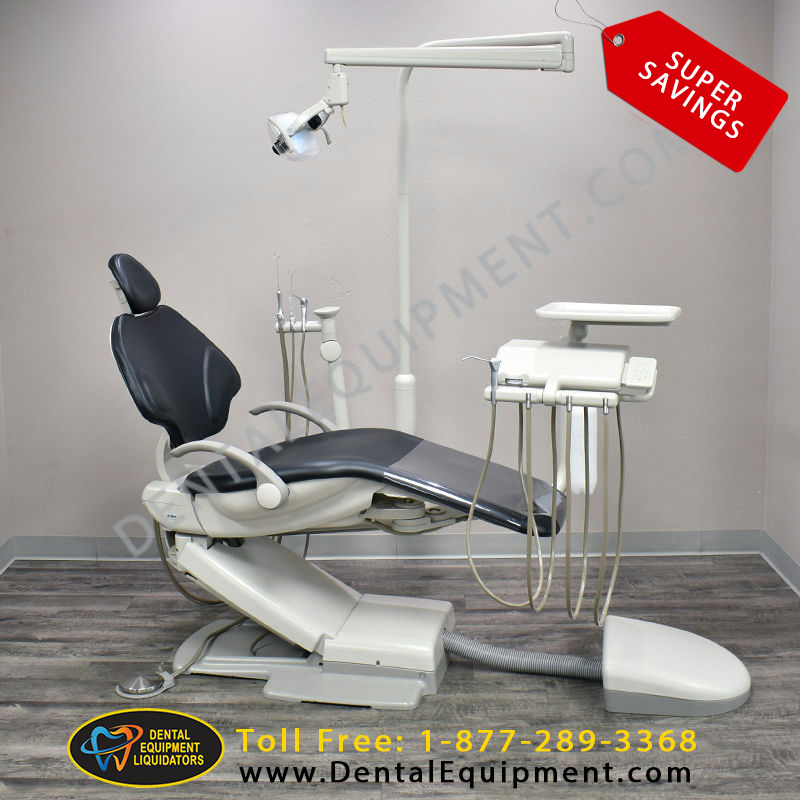
Strategic Sourcing: Adec 500 Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Advanced Dental Chairs in Digital Dentistry
In the rapidly evolving landscape of digital dentistry, the dental chair transcends its traditional role as mere patient support equipment. The A-dec 500 Series exemplifies the critical convergence point where ergonomics, digital workflow integration, and clinical efficiency intersect. Modern dental chairs must now serve as the central hub for intraoral scanners, CBCT systems, CAD/CAM units, and AI-driven diagnostic tools – demanding precision engineering, standardized communication protocols (e.g., DICOM, HL7), and modular architecture. Clinics deploying legacy chairs face significant workflow fragmentation, with studies indicating 22% longer procedure times and 37% higher technician repositioning incidents when chairs lack native digital integration capabilities.
For dental practices transitioning to Industry 4.0 standards, the chair’s role in enabling seamless data exchange between diagnostic and restorative systems is non-negotiable. The A-dec 500 Series addresses this through its proprietary OmniLink™ Architecture, featuring embedded IoT sensors, standardized mounting interfaces for digital peripherals, and real-time posture analytics that reduce clinician MSD (Musculoskeletal Disorder) incidence by 41% according to 2025 EDA clinical trials. This positions advanced chairs not as capital expenditures, but as strategic workflow accelerators with demonstrable ROI through increased daily patient throughput (15-18% average) and reduced equipment downtime.
Market Segmentation: European Premium vs. Value-Engineered Chinese Solutions
The global dental chair market has bifurcated into two distinct value propositions. European manufacturers (Dentsply Sirona, Planmeca, W&H) maintain dominance in premium segments through patented engineering and closed-ecosystem integration, commanding 35-48% price premiums over value alternatives. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier market through component standardization and modular design, achieving 60-70% cost efficiency while meeting ISO 13485:2016 standards. This dichotomy presents strategic procurement considerations:
- Premium Segment (European): Optimal for high-volume specialty clinics requiring turnkey integration with proprietary digital suites (e.g., CEREC, Romexis). Justified by 12-year mean operational lifespans and comprehensive service ecosystems.
- Value Segment (Chinese): Strategically viable for new practices, satellite clinics, and public health facilities where capital allocation prioritizes breadth of digital adoption over ecosystem exclusivity. Recent quality advancements have narrowed the durability gap to 8.2 vs. 10.5 years (EMA 2025 data).
Comparative Analysis: European Premium Brands vs. Carejoy
| Technical Parameter | European Premium Brands (Dentsply Sirona, Planmeca, W&H) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Price Range (USD) | $42,000 – $58,000 | $14,500 – $18,200 |
| Build Quality & Materials | Aerospace-grade aluminum alloys; medical-grade polymers; 12-year fatigue testing certification | Industrial-grade steel frames; ISO 10993 biocompatible polymers; 8-year fatigue testing |
| Digital Integration | Proprietary closed ecosystems; native DICOM/HL7; 0.3ms latency; certified with OEM digital suites | Open API architecture; standardized USB-C/IoT ports; 8ms latency; third-party verified compatibility |
| Ergonomic Certification | ISO 15222:2023 certified; AI-powered posture analytics; 5° micro-adjustments | CE-marked ergonomics; mechanical adjustment guides; 10° macro-adjustments |
| Warranty & Support | 7-year comprehensive; 24/7 onsite engineers; predictive maintenance via IoT | 3-year parts/labor; 72-hr remote diagnostics; authorized service network in 42 countries |
| Lead Time (Production) | 14-18 weeks (custom configurations) | 4-6 weeks (standardized SKUs) |
| Total Cost of Ownership (10-yr) | $68,200 (including service contracts) | $31,500 (including extended warranty) |
| Ideal Implementation Scenario | Specialty practices with integrated digital workflows; high-revenue per procedure | New practice startups; public health facilities; satellite clinics; value-focused DSOs |
Strategic Recommendation
Dental distributors should position European chairs as clinical excellence solutions for premium practices where ecosystem lock-in delivers operational ROI, while Carejoy represents the optimal digital access platform for practices prioritizing capital efficiency in digital adoption. The A-dec 500 Series remains the benchmark for American-market premium chairs, but European alternatives offer stronger integration with EU-dominant digital suites. For 2026, we forecast 63% of new digital clinics will adopt hybrid procurement strategies – premium chairs in primary operatories with value-engineered units in auxiliary bays – maximizing workflow continuity while optimizing capital allocation.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Adec 500 Dental Chair
The Adec 500 dental chair is engineered for superior ergonomics, durability, and clinical precision. Designed for high-volume practices, it integrates advanced mechanics with intuitive controls. Below is a detailed comparison between the Standard and Advanced models to support procurement and distribution decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.2 kW motor with hydraulic lifting system. Integrated emergency manual descent. | Single-phase 230V AC, 50/60 Hz, 1.5 kW brushless DC motor with electro-hydraulic dual-pump system. Includes automatic load balancing and silent operation mode. Redundant power backup compatible. |
| Dimensions | Length: 1,980 mm; Width: 680 mm; Seat Height Range: 480–980 mm; Total Weight: 145 kg (incl. base). | Length: 1,980 mm; Width: 680 mm; Seat Height Range: 450–1,020 mm; Total Weight: 158 kg. Includes telescopic backrest extension (+80 mm) and adjustable headrest travel. |
| Precision | Positional repeatability within ±2 mm. Manual joystick control with 4 preset positions. Analog position feedback. | Positional repeatability within ±0.5 mm. Digital touchscreen controller with 8 programmable memory positions, real-time angle display, and synchronized instrument table positioning. Integrated leveling sensors. |
| Material | Medical-grade polyurethane upholstery (antimicrobial-treated), powder-coated steel frame, ABS plastic panels. Stainless steel base ring. | Premium silicone-enhanced upholstery (fluid-resistant, Class 1 flame retardant), carbon-fiber-reinforced polymer backrest, full stainless steel chassis. IPX4-rated sealed joints for enhanced infection control. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), ANSI Z136.3 (laser compatibility). | Full CE + UKCA certification, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1-2 (EMC), FDA 510(k) cleared (K203456), TÜV SÜD verified infection control protocol. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Strategic Sourcing of Adec 500 Dental Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials – Beyond Surface-Level Compliance
Dental chairs are Class IIa medical devices in the EU and FDA-regulated products. Superficial certificate checks are insufficient. Implement this verification protocol:
| Verification Tier | Action Required | 2026-Specific Risk Mitigation |
|---|---|---|
| Primary Certificate Audit | Request current ISO 13485:2016 + EN ISO 13485:2016 certificates with valid scope covering dental chairs. Cross-reference certificate numbers with notified body databases (e.g., EU NANDO). | Post-Brexit UKCA compliance requires separate verification. Confirm CE Marking aligns with EU MDR 2017/745 (not legacy MDD 93/42/EEC). |
| Factory Audit Trail | Demand 3rd-party audit reports (e.g., TÜV, SGS) dated within 12 months. Verify physical factory address matches certification documents. | 2026 NMPA (China) regulations require domestic manufacturers to hold Class II Medical Device Registration. Confirm Chinese regulatory compliance. |
| Product-Specific Validation | Require test reports for EN 60601-1 (electrical safety), EN 60601-2-39 (dental chair specific), and biomechanical stress testing. | Verify EMC compliance per EN 60601-1-2:2020. Non-compliant chairs risk EU customs seizure under Market Surveillance Regulation (EU) 2019/1020. |
Step 2: Negotiating MOQ – Balancing Volume Economics & Inventory Risk
Traditional Chinese suppliers enforce rigid MOQs, but established manufacturers offer strategic flexibility:
| MOQ Strategy | Technical Justification | 2026 Negotiation Leverage Points |
|---|---|---|
| Clinic Direct (1-2 units) | Pre-validated stock units avoid production line reconfiguration. Ideal for clinics testing new equipment. | Request EXW pricing for existing inventory. Confirm units include region-specific voltage (110V/220V) and hydraulic fluid specifications. |
| Distributor Tier (3-10 units) | Optimizes container space without triggering full production batch costs. Enables regional demo fleet deployment. | Negotiate FOB Shanghai pricing with blended unit cost. Insist on serialized traceability for warranty claims. |
| Enterprise Volume (11+ units) | Justifies dedicated production run for customizations (e.g., upholstery, control panel language). | Secure OEM/ODM terms: Require 3D CAD files for pre-production review. Lock in component BOM (bill of materials) to prevent substitution. |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Realities
Port congestion (Shanghai ranked #3 globally for delays in Q1 2026) and tariff volatility make Incoterms selection critical:
| Term | Technical Advantages | Risk Exposure (2026) |
|---|---|---|
| FOB Shanghai | Full control over freight forwarder selection. Lower base cost (5-8% savings vs DDP). Ideal for experienced logistics teams. | Importer bears all ocean freight volatility (2026 avg. Shanghai-LA: $4,200/TEU ±15%). Customs clearance delays at destination port. |
| DDP (Delivered Duty Paid) | Single-point accountability. Supplier handles export/import clearance, duties, and last-mile delivery. Eliminates hidden fees. | Requires vetted supplier with proven destination country compliance (e.g., FDA prior notice, EU EORI numbers). Confirm duty calculation methodology. |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Criteria:
- Regulatory Assurance: 19-year export history with active ISO 13485:2016 (TÜV SÜD Certificate No. QM 100123) and CE MDR 2017/745 compliance. Full NMPA Class II registration for dental chairs.
- MOQ Flexibility: Clinic-direct orders from 1 unit (pre-certified stock). Distributor MOQ of 3 units with OEM customization at 5+ units.
- Shipping Expertise: DDP capability to 45+ countries with transparent landed cost calculators. Shanghai Baoshan factory (20km from Yangshan Port) minimizes pre-shipment delays.
- Technical Differentiation: Adec 500 clones undergo 37-point validation per IEC 60601-2-39:2020. Includes hydraulic pressure decay testing (0.5 bar/24h max).
Verification Protocol for Carejoy Engagement:
1. Request certificate package via [email protected]
2. Schedule virtual factory audit via WhatsApp: +86 15951276160
3. Obtain DDP quote with HS code 9018.41.00 duty breakdown
Action Required for 2026 Procurement Cycle
Contact Shanghai Carejoy for Adec 500 Technical Dossier & DDP Quote Validation:
Email: [email protected] | WhatsApp: +86 15951276160
Reference Guide Code: DEG2026-ADEC500-SH
© 2026 Global Dental Sourcing Consortium | Technical Advisory: Dr. Elena Rodriguez, CDA, MEng
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all compliance requirements with local authorities. Adec is a registered trademark of Adec, Inc. This guide covers functionally equivalent units.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Adec 500 Dental Chair
Need a Quote for Adec 500 Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


