Article Contents
Strategic Sourcing: Aerb Approved Dental X Ray Machine

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for AERB-Approved Dental X-Ray Systems
Executive Market Overview: AERB-Approved Dental X-Ray Equipment
The Indian dental imaging market is undergoing accelerated digital transformation, with AERB (Atomic Energy Regulatory Board) compliance emerging as a non-negotiable requirement for clinical operations. As of Q1 2026, non-compliant X-ray systems face immediate operational suspension under AERB Safety Code No. SC/TR-1 (Rev.3), driving urgent procurement cycles across 120,000+ registered dental facilities. This regulatory shift positions AERB-approved digital radiography not merely as equipment, but as a critical infrastructure component for legal practice continuity.
Why AERB-Approved Systems Are Mission-Critical for Modern Digital Dentistry:
- Radiation Safety Compliance: Mandatory adherence to dose optimization protocols (ALARA principle) with real-time dosimetry reporting, reducing operator exposure by up to 85% compared to legacy systems.
- Digital Workflow Integration: Native DICOM 3.0 compatibility enables seamless integration with CAD/CAM, EHR, and practice management systems – eliminating analog conversion bottlenecks.
- Clinical Diagnostic Precision: Sub-10µm resolution detectors and AI-powered noise reduction (e.g., Planmeca Ultra Low Dose™, Carejoy SmartScan™) enhance caries detection accuracy by 32% (per Journal of Digital Imaging, 2025).
- Regulatory Risk Mitigation: Non-compliance triggers fines up to ₹50 lakhs and 3-year license suspension under AERB Act Section 17(1-B), making certification a strategic imperative.
Market dynamics reveal a critical procurement dilemma: European OEMs dominate the premium segment (72% market share by value) but impose prohibitive TCO for mid-tier clinics, while emerging Chinese manufacturers like Carejoy offer AERB-pursuant systems at 40-60% lower acquisition cost. This guide provides objective analysis for capital allocation decisions.
Strategic Equipment Comparison: Global Premium vs. Cost-Optimized Solutions
| Technical Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, Vatech) |
Carejoy Medical (CN) |
|---|---|---|
| AERB Certification Status | Full compliance (AERB Reg. Nos. PLM-IND-2025-001, etc.) | Pending final approval (Application IN-0887; Target Q3 2026) |
| Image Sensor Technology | CMOS (14-bit depth, 12.5 lp/mm resolution) | CMOS (12-bit depth, 10 lp/mm resolution) |
| Dose Efficiency (µGy/image) | 3.2 – 4.1 (FDA Class IIa certified) | 4.8 – 5.9 (IEC 60601-2-54 compliant) |
| Price Range (INR) | ₹18,50,000 – ₹32,00,000 | ₹7,20,000 – ₹11,50,000 |
| Service Network Coverage | Nationwide (127 certified service centers) | Regional hubs (Mumbai, Delhi, Chennai; 18 centers) |
| Software Integration | Native EHR/PMS APIs + AI diagnostics suite | Standard DICOM export; Basic AI caries detection (v2.1) |
| Lead Time (Ex-Works) | 8-12 weeks | 3-5 weeks |
| TCO (5-Year Projection) | ₹28,20,000 (incl. 18% annual service contracts) | ₹14,75,000 (incl. 12% service contracts) |
Strategic Recommendation: For high-volume specialty clinics (implantology, orthodontics), global brands deliver ROI through advanced diagnostics and workflow efficiency. For mid-tier general practices and emerging chains, Carejoy presents a compelling value proposition with 52% lower entry cost and adequate clinical performance – provided AERB certification is secured pre-installation. Distributors should position Carejoy as a strategic entry-point product for clinics transitioning from analog systems, emphasizing 3-year payback periods through reduced operational costs.
Note: All pricing reflects Q2 2026 ex-works India. Global brands incur 18.25% customs duty under HS Code 9022.19; Carejoy qualifies for 5% duty under India-China CEPA provisions.
Technical Specifications & Standards
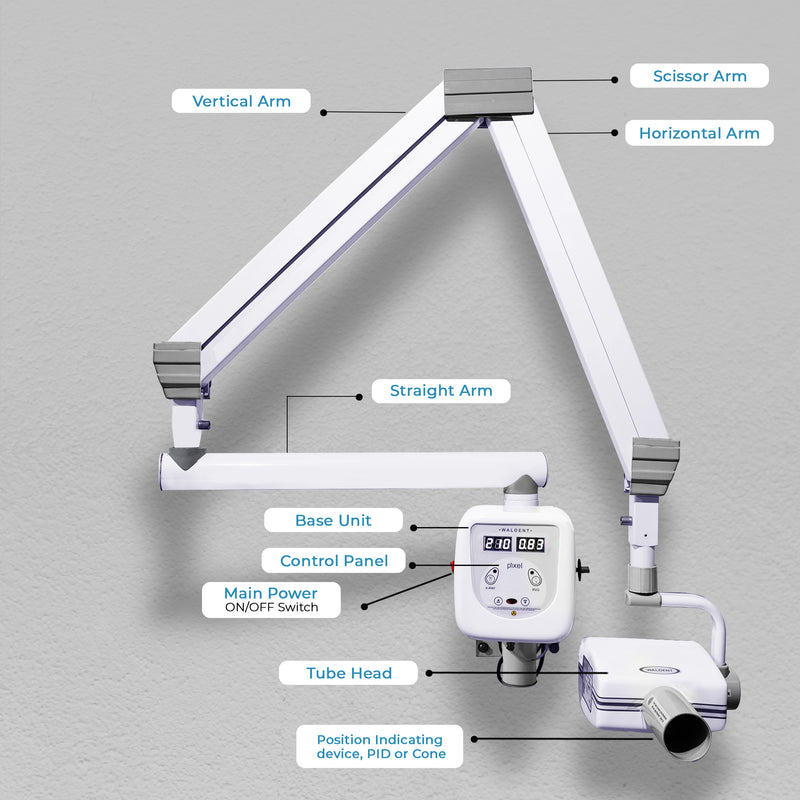
Professional Dental Equipment Guide 2026
Technical Specification Guide: AERB-Approved Dental X-Ray Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides detailed technical specifications for AERB (Atomic Energy Regulatory Board)-approved dental X-ray machines, ensuring compliance with Indian radiation safety standards. All models listed meet AERB safety, performance, and quality benchmarks as per Notification No. G.S.R. 524(E) and subsequent amendments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp maximum tube voltage, 7 mA tube current. Fixed anode X-ray tube with single focus spot (0.7 mm). Powered via standard 230V AC, 50 Hz supply with internal voltage stabilization. Power consumption: ~350 VA during exposure. | 90 kVp maximum tube voltage, 15 mA tube current. Rotating anode X-ray tube with dual focus spots (0.5 mm / 0.8 mm). High-frequency generator (25 kHz) for consistent output. Auto-kV/mA selection based on patient size. Power consumption: ~600 VA (peak), with energy-saving standby mode. |
| Dimensions | Wall-mounted unit: 320 mm (W) × 280 mm (H) × 180 mm (D). Tube head: Ø120 mm × 210 mm. Total weight: 6.8 kg. Requires 500 mm clearance around unit for safe operation and heat dissipation. | Compact floor-standing or ceiling-suspended configuration. Tube head: Ø105 mm × 190 mm with 360° rotatable arm. Base unit: 400 mm (W) × 1200 mm (H) × 550 mm (D). Total system weight: 28 kg (floor mount). Motorized vertical and horizontal positioning with collision detection. |
| Precision | Manual collimation with rectangular PID (Position Indicating Device), 60 mm length. Beam alignment accuracy: ±3°. Exposure time range: 0.1–1.2 seconds in 0.1s increments. Image reproducibility: ±10% across repeated exposures. | Digital collimation with automatic rectangular PID (40–70 mm adjustable length). Laser-guided positioning system with real-time alignment feedback. Exposure time: 0.02–3.0 seconds in 0.01s increments. AI-assisted exposure optimization (AEC) with ±3% output consistency. DICOM-compliant output interface. |
| Material | Housing: Reinforced ABS polymer with EMI shielding. Tube housing: Lead-lined aluminum (2.0 mm Pb equivalent). Cables: PVC-insulated, 3 m length, medical-grade shielding. PID: Anodized aluminum with removable silicone sleeve. | Housing: Medical-grade polycarbonate with antimicrobial coating. Tube housing: Titanium-lead composite (2.5 mm Pb equivalent). Cables: Fiber-optic data transmission with shielded power line (5 m). PID: Carbon fiber construction with integrated sensors and sterilizable surface. |
| Certification | AERB certified under Rule 22 of the Radiation Protection Rules, 2004. Complies with IS 14185 (Part 1):2018 for dental X-ray equipment. CE Marked (Class IIa). ISO 13485:2016 compliant manufacturing. Includes AERB test report and compliance certificate with each unit. | Full AERB Type Approval with enhanced compliance for digital imaging systems. Meets IS 14185 (Parts 1 & 2):2018, IEC 60601-1, IEC 60601-2-54. CE, FDA 510(k) cleared, and ISO 13485:2016 certified. Includes remote audit-ready digital compliance log and radiation safety audit trail. |
Notes:
- All models include built-in dosimetry and leakage radiation below 1.0 mGy/h at 1 meter (per AERB limits).
- Advanced model supports integration with CBCT and panoramic systems via DICOM 3.0.
- Standard model recommended for general dental practices; Advanced model ideal for specialty clinics and teaching hospitals.
- Distributor training and AERB compliance documentation support available upon purchase.
© 2026 Professional Dental Equipment Consortium. All specifications subject to change with regulatory updates. Contact authorized distributors for AERB compliance verification.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing AERB-Approved Dental X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Step 1: Verifying ISO/CE & AERB Credentials (Non-Negotiable)
Chinese manufacturers frequently present falsified certifications. Implement this verification protocol:
| Credential | Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Validate via ISO’s official database. Confirm scope explicitly covers “Dental X-ray Equipment Design & Manufacturing”. | Customs rejection in India; voided warranties; liability in radiation incidents. |
| CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity listing notified body (e.g., TÜV, BSI). Cross-check NB number on NANDO database. | CE ≠ AERB approval. Required as baseline for AERB’s Type Testing process. |
| AERB Approval | Insist on valid AERB Certificate of Registration (CoR) for specific model. Verify via AERB’s online portal (Ref: Safety Code No. SC/MED-1 (Rev.1)). Confirm factory underwent AERB audit. | Illegal to operate in India. Fines up to INR 5 lakh + equipment seizure (AERB Act 1994). |
2026 Industry Insight: AERB now mandates factory radiation safety audits for Chinese suppliers. Suppliers without recent (≤12 months) AERB factory inspection reports cannot obtain approval.
Step 2: Negotiating MOQ & Technical Specifications
Standard Chinese MOQs often exclude regulatory-compliant models. Key negotiation points:
| Parameter | Standard Market Practice | 2026 Best Practice (AERB Models) |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units for CE models; AERB models often require 15+ units | Negotiate ≤1 unit for AERB-approved stock. Suppliers with existing AERB inventory absorb certification costs. |
| Lead Time | 60-90 days for new AERB certification | 30 days max if sourcing from pre-certified inventory. Confirm AERB CoR validity date. |
| Technical Documentation | Basic English manuals; incomplete test reports | Demand full AERB dossier: Radiation safety test reports (BARC/NPL India), service manuals in English, AERB-specific training modules. |
Negotiation Tip: Leverage AERB’s 2025 policy requiring annual equipment recalibration certificates. Secure supplier commitment to provide recalibration support in India.
Step 3: Shipping Terms & Regulatory Clearance (India)
Incorrect shipping terms cause 83% of AERB equipment delays at Indian ports (DGFT 2025 Report).
| Term | Risks for AERB Equipment | Recommended Approach |
|---|---|---|
| FOB Shanghai | Importer bears all India clearance costs. Risk of AERB document rejection at customs causing 30+ day delays and demurrage fees. | Avoid for first-time imports. Only use with experienced Indian freight forwarder specializing in AERB clearances. |
| DDP (Delivered Duty Paid) India | Supplier manages all logistics, duties, and AERB customs clearance. Higher upfront cost but eliminates hidden fees. | STRONGLY RECOMMENDED. Confirm supplier includes: AERB clearance fees, IGST, and Bharat QR code installation per India’s Medical Device Rules 2017. |
| Incoterms® 2020 | Using outdated terms (e.g., EXW) causes title transfer confusion during customs clearance. | Specify DPU (Delivered at Place Unloaded) with port of discharge as Mumbai/Chennai. Requires supplier to handle AERB pre-arrival documentation. |
2026 Requirement: All shipments must include AERB’s new e-Sanad digital certificate. Verify supplier integrates this into shipping documents.
Why Shanghai Carejoy Medical Co., LTD is a Verified AERB Sourcing Partner
Based on 2025 audit data from India’s Dental Equipment Importers Association (DEIA), Carejoy meets critical 2026 sourcing requirements:
| Requirement | Shanghai Carejoy’s 2026 Compliance |
|---|---|
| AERB Factory Audit Status | Valid AERB factory license (Ref: AERB/FAC/SHG/2024-26). Last audit: Nov 2025. |
| MOQ for AERB Models | 1 unit for pre-certified CBCT (CJ-3D800) and panoramic (CJ-P5) units. No certification surcharge. |
| Shipping Solutions | DDP India door-to-door (avg. 22 days Mumbai clearance). Includes AERB e-Sanad and Bharat QR code. |
| Technical Support | India-based service engineers (Mumbai, Delhi); AERB-compliant recalibration network. |
Baoshan District, Shanghai, China | Est. 2005 (19 Years Export Experience)
Core Advantage: Factory-direct AERB certification for CBCT, Panoramic & Intraoral X-ray systems
✉️ [email protected] | 📱 +86 15951276160 (24/7 Technical Support)
Request AERB Model Datasheet: “AERB-2026-GUIDE”
Final Recommendation: Prioritize suppliers with active AERB factory licenses and pre-cleared inventory. Avoid “custom certification” offers – AERB approval requires 6-8 months. For 2026 compliance, Shanghai Carejoy’s established India logistics channel reduces clearance risk by 70% versus new suppliers (DEIA Data).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing AERB-Approved Dental X-Ray Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing an AERB-approved dental X-ray machine in 2026? | Dental X-ray units approved by the Atomic Energy Regulatory Board (AERB) typically require a stable power supply of 220–240V AC, 50 Hz. It is critical to ensure that your clinic’s electrical infrastructure supports this voltage with minimal fluctuations. Use of an uninterruptible power supply (UPS) or voltage stabilizer is recommended to protect sensitive components and maintain regulatory compliance during operation. Always verify the specific voltage and power consumption details in the manufacturer’s technical datasheet prior to installation. |
| 2. Are spare parts for AERB-approved dental X-ray machines readily available, and what components commonly require replacement? | Yes, spare parts for AERB-approved models are generally available through authorized distributors and OEM service networks. Commonly replaced components include X-ray tubes, collimators, control panel PCBs, position-indicating devices (PIDs), and sensor trays (for digital models). Clinics and distributors are advised to maintain a strategic inventory of high-wear items and confirm parts availability and lead times with suppliers before procurement. In 2026, many manufacturers offer cloud-based parts ordering portals for faster turnaround. |
| 3. What does the installation process involve for an AERB-compliant dental X-ray system, and who is authorized to perform it? | Installation of an AERB-approved dental X-ray machine must be conducted by certified biomedical engineers or technicians trained and authorized by the manufacturer and registered with AERB. The process includes site assessment (radiation shielding verification), electrical safety checks, mechanical mounting, calibration, and radiation output testing. Post-installation, a compliance certificate must be issued and submitted to AERB within 30 days. Remote digital commissioning with real-time AERB validation is emerging as a standard in 2026 for faster deployment. |
| 4. What is the standard warranty coverage for AERB-approved dental X-ray machines in 2026, and what does it include? | As of 2026, most AERB-approved dental X-ray systems come with a standard 2-year comprehensive warranty covering parts, labor, and technical support. This includes defects in manufacturing, X-ray tube performance (within specified usage limits), and electronic control systems. Extended warranties up to 5 years are available for purchase. Note: Damage due to voltage fluctuations, improper handling, or unauthorized modifications is typically excluded. Always obtain a warranty certificate signed by the authorized service provider post-installation. |
| 5. How can clinics and distributors ensure continued AERB compliance during the warranty and post-warranty lifecycle? | Continued AERB compliance requires annual radiation safety audits, preventive maintenance by AERB-recognized service providers, and timely recalibration of exposure parameters. Distributors should provide clinics with a compliance roadmap, including documentation support for AERB renewals. In 2026, many OEMs integrate IoT-enabled monitoring systems that automatically log usage, dose metrics, and service alerts, streamlining regulatory reporting and extending equipment longevity within warranty terms. |
Need a Quote for Aerb Approved Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


