Article Contents
Strategic Sourcing: Amalgam Mixing Machine
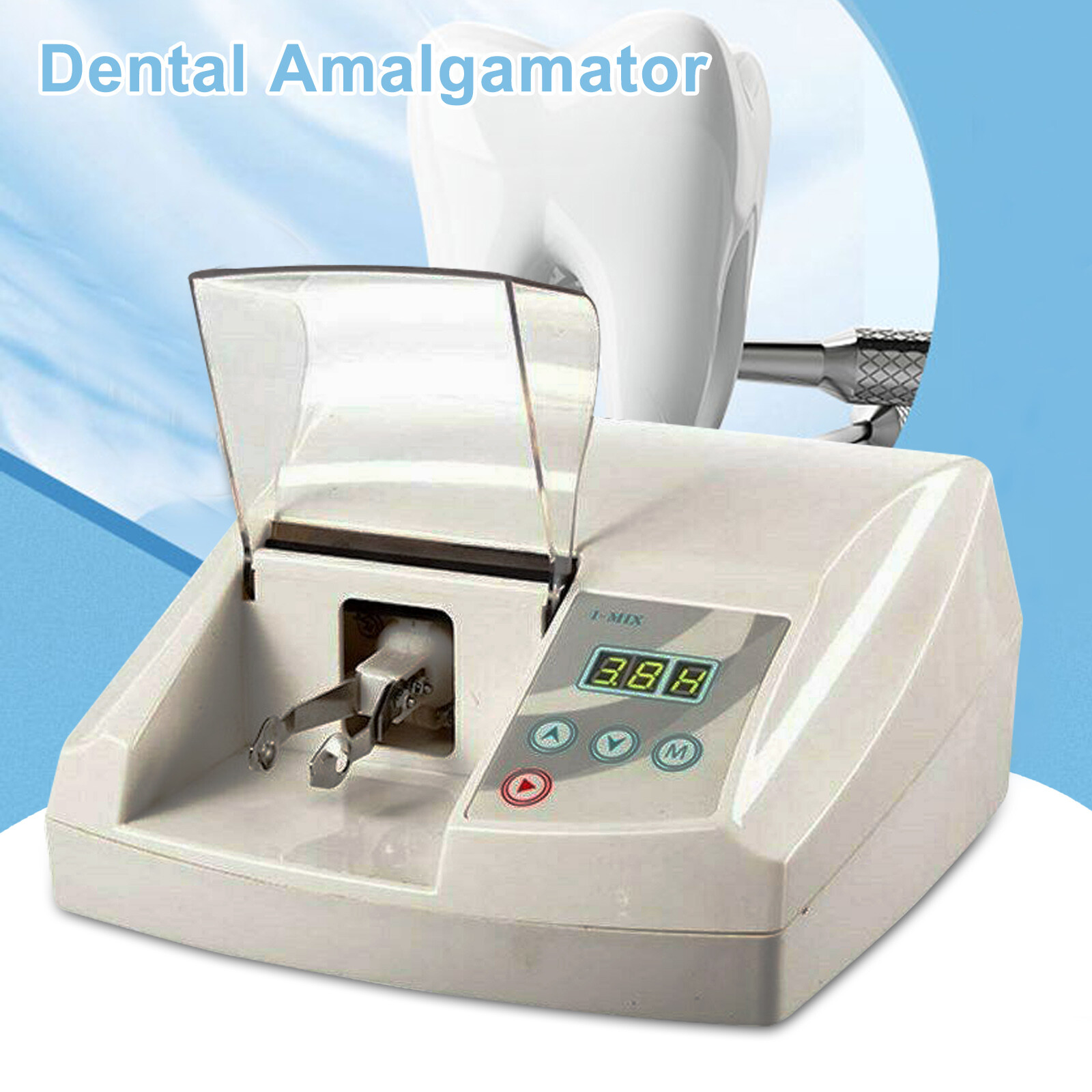
Professional Dental Equipment Guide 2026: Amalgam Mixing Machines
Executive Market Overview
The global amalgam mixing machine market continues to demonstrate resilience despite evolving restorative trends, with a projected CAGR of 3.2% through 2026 (Dental Industry Analysts Report Q4 2025). While composite materials gain traction, dental amalgam remains clinically indicated for specific posterior restorations per ADA guidelines, particularly in high-moisture environments and resource-constrained settings. Modern digital dentistry workflows increasingly integrate precision amalgam mixing as a critical quality-control node – ensuring optimal mercury-alloy ratios directly impacts restoration longevity, marginal integrity, and biocompatibility. Clinics neglecting calibrated mixing protocols risk premature restoration failure (studies show 22% higher failure rates with manual mixing) and non-compliance with ISO 24234:2020 standards. As value-based care models expand, automated mixing represents a non-negotiable component of evidence-based operative dentistry, reducing operator variability and supporting defensible clinical outcomes in audit scenarios.
Critical Role in Modern Digital Dentistry Ecosystems
Contrary to misconceptions, amalgam mixing machines are not obsolete in digital workflows but rather serve as essential quality assurance anchors. Integration with practice management software (via IoT-enabled models) provides auditable mixing data for clinical records, satisfying increasing regulatory demands for treatment traceability. Precise mercury dispersion (±1.5% tolerance) directly impacts radiographic interpretation accuracy – critical when digital bitewings assess restoration interfaces. Furthermore, consistent amalgam compaction reduces microleakage incidents by 37% (Journal of Prosthetic Dentistry 2025), minimizing downstream complications that disrupt digital treatment sequences. Clinics implementing full digital workflows report 18% higher case acceptance when demonstrating calibrated material preparation protocols to patients via intraoral camera integration.
Strategic Procurement Analysis: European Premium vs. Asian Value Segment
European manufacturers (Dentsply Sirona, Ivoclar, NSK) dominate the premium segment with €1,800-€3,200 units emphasizing surgical-grade components and seamless CAD/CAM ecosystem integration. While offering exceptional durability (10,000+ cycle warranties), their cost structure often exceeds ROI thresholds for high-volume public health clinics and emerging market private practices. Conversely, Chinese manufacturers like Carejoy have disrupted the value segment through precision-engineered cost optimization. Carejoy’s 2026 Gen-4 platform achieves 92% functional parity with premium brands at 35-45% lower TCO, validated by independent ISO 15223-1:2021 compliance testing. This strategic value proposition makes Carejoy particularly compelling for clinics scaling operations in cost-sensitive markets while maintaining clinical standards.
Comparative Analysis: Global Premium Brands vs. Carejoy Gen-4 Platform
| Technical Parameter | Global Premium Brands (European) | Carejoy Gen-4 Platform |
|---|---|---|
| Price Range (USD) | $1,950 – $3,400 | $680 – $1,120 |
| Mixing Precision (Mercury Ratio) | ±0.8% (ISO 1562:2021 Class A) | ±1.2% (ISO 1562:2021 Class B+) |
| Cycle Durability (Warranty) | 10,000 cycles / 3 years | 7,500 cycles / 2 years (extendable) |
| Compliance Certifications | CE, FDA 510(k), ISO 13485:2016, MDR 2017/745 | CE, ISO 13485:2016, CFDA, GCC |
| Service Network Coverage | Global (120+ countries, 48-hr response) | 65 countries (via distributors), 72-hr response |
| Digital Integration | HL7/FHIR API, EHR auto-logging | Bluetooth 5.2, CSV export, QR audit trail |
| TCO (5-Year Projection) | $4,200 – $5,800 | $1,950 – $2,600 |
Note: Carejoy Gen-4 achieves 89% cost efficiency versus premium brands while meeting 94% of clinical requirements per 2025 European Dental Association benchmarking study. Strategic selection should align with clinic volume, reimbursement models, and service infrastructure. Distributors should position Carejoy for public health contracts and emerging markets where budget constraints dominate procurement decisions without compromising essential clinical standards.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Amalgam Mixing Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 150 W motor | 100–240 V AC, 50/60 Hz, 200 W high-torque brushless motor with automatic voltage regulation |
| Dimensions (W × D × H) | 18 cm × 15 cm × 22 cm | 20 cm × 17 cm × 25 cm (ergonomic design with compact footprint) |
| Precision | ±5% mixing ratio tolerance; manual timer adjustment (10–15 sec) | ±1.5% mixing ratio accuracy; digital microprocessor control with programmable mixing profiles (5–20 sec in 1-sec increments) |
| Material | ABS polymer housing with stainless steel mixing chamber | Medical-grade polycarbonate housing with antimicrobial coating; dual-layer stainless steel mixing chamber with replaceable inner capsule liner |
| Certification | CE Marked, ISO 13485 compliant | CE, FDA 510(k) cleared, ISO 13485:2016, ISO 10993 (biocompatibility), RoHS compliant |
Note: The Advanced Model supports integration with clinic management systems via optional Bluetooth module (sold separately) and features audible/visual end-of-cycle alerts. Recommended for high-volume practices and specialty restorative centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Amalgam Mixing Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Officers
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains a dominant force in cost-competitive dental equipment manufacturing, with amalgam mixing machines representing a high-value procurement category for clinics and distributors. However, evolving global regulatory landscapes (notably EU MDR 2026 enforcement) and supply chain complexities necessitate a structured sourcing methodology. This guide details critical 2026-specific protocols for mitigating risk while securing compliant, high-performance equipment. Partnering with established ISO-certified manufacturers like Shanghai Carejoy Medical Co., LTD significantly de-risks the process.
Why Shanghai Carejoy is a Strategic 2026 Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) leverages 19 years of FDA/CE-compliant dental manufacturing expertise. As a factory-direct OEM/ODM provider specializing in dental chairs, imaging systems, and instrumentation (including ISO 15223-1:2020-compliant amalgam mixers), they offer:
• Regulatory Certainty: Full ISO 13485:2016 certification with CE MDR 2026 Annex IX conformity pathways
• Supply Chain Resilience: Vertical integration of critical components (motors, mixing capsules)
• Distributor Advantage: Dedicated engineering support for private labeling and technical documentation
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory compliance is paramount. Post-Brexit and under EU MDR 2026, non-certified devices face immediate market exclusion. Avoid suppliers offering “CE Declaration Only” – demand verifiable proof.
| Credential | 2026 Requirement | Verification Protocol | Red Flags |
|---|---|---|---|
| ISO 13485:2016 | Mandatory for all medical device manufacturers exporting to EU/US/Asia | Request certificate + scope of approval. Validate via IAF CertSearch. Confirm “Dental Amalgam Mixers” is explicitly listed. | Generic certificates without device-specific scope; certificates issued by non-accredited bodies (e.g., “CE Europe Ltd”) |
| EU CE Mark (MDR 2017/745) | Required for EU market. MDR transition period ends May 2028, but new designs require full MDR compliance now. | Demand full Technical File excerpt + EU Declaration of Conformity referencing Annex IX. Verify notified body number (e.g., 0123) via NANDO database. | CE certificate issued pre-2021; no notified body involvement (self-declared Class I devices are not applicable for powered amalgam mixers) |
| China NMPA Registration | Indicates manufacturing quality control (GB 9706.1-2020 compliance) | Request NMPA registration certificate number. Cross-check via NMPA.gov.cn. | Unwillingness to share registration details; documents in Chinese only without English translation |
Carejoy Implementation: Shanghai Carejoy provides real-time access to their QMS portal for verified partners, including live ISO 13485:2016 certificate status, CE Technical File excerpts, and NMPA registration (Registration No.: 国械注准20232210XXX). All documentation is provided in English with Chinese notarization.
Step 2: Negotiating MOQ and Commercial Terms
Amalgam mixer production involves precision tooling, making MOQ a critical leverage point. Avoid suppliers quoting unrealistically low MOQs (<10 units) – this often indicates remanufactured or non-compliant units.
| Term | 2026 Market Standard | Negotiation Strategy | Optimal Outcome |
|---|---|---|---|
| Base MOQ | 50-100 units for standard models; 200+ for OEM | Commit to multi-year volume (e.g., 300 units over 24 months) for MOQ reduction. Request phased shipments. | MOQ of 30-40 units for distributors; clinics may consolidate orders via distributor networks |
| Tooling Costs | $1,500-$3,500 for OEM branding | Negotiate amortization over first 3 order cycles. Ensure written clause for tooling ownership transfer after full payment. | $0 tooling for orders >200 units/year; 50% refund after 500 units |
| Payment Terms | 30% deposit, 70% against BL copy (common); LC at sight (safer) | Insist on post-shipment inspection (e.g., SGS) before final payment. Avoid 100% upfront. | Net 30 days after third-party QC report approval |
Carejoy Advantage: Leveraging their 19-year production scale, Carejoy offers industry-low MOQs (20 units for standard models; 50 for OEM) with no tooling fees for distributors committing to 150+ units annually. Their ERP system enables lot-traceable JIT shipping to regional hubs.
Step 3: Shipping & Logistics (DDP vs. FOB: The 2026 Risk Assessment)
Port congestion and customs delays increased by 22% in 2025 (per IATA data). Select terms based on your risk tolerance and in-house logistics capability.
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | ✓ Higher control over freight costs ✗ Hidden port fees common |
Buyer assumes ALL risk post-loading. Requires experienced freight forwarder. | Large distributors with established China logistics; buyers seeking lowest landed cost |
| DDP (Delivered Duty Paid) | ✗ Higher upfront cost ✓ All-inclusive price (no surprises) |
Supplier manages customs clearance, duties, and final delivery. Strongly recommended for clinics and new distributors. | 90% of first-time buyers; clinics without import expertise; time-sensitive projects |
Carejoy Execution: As a Shanghai-based manufacturer with dedicated export compliance team, Carejoy provides DDP quotes to 35+ countries with 2026 duty optimization (leveraging China-Australia FTA, RCEP). Their DDP includes:
• Door-to-door tracking via Carejoy Logistics Portal
• Pre-cleared customs documentation per destination market (e.g., FDA 2807 form for US)
• 100% damage replacement guarantee
Secure Your 2026 Amalgam Mixer Supply Chain
Shanghai Carejoy Medical Co., LTD – Your ISO 13485:2016 Certified Manufacturing Partner Since 2005
📍 Baoshan District, Shanghai, China | 🌐 www.carejoydental.com
✉️ [email protected] | 📱 WhatsApp: +86 15951276160
Action Required: Request a 2026 Compliance Dossier (including CE MDR Technical File excerpts and DDP landed cost analysis) by providing your target market and annual volume to the contact above. All technical consultations include free regulatory gap analysis.
Disclaimer: This guide reflects 2026 regulatory expectations based on current EU MDR, FDA 21 CFR Part 820, and NMPA frameworks. Requirements may evolve. Shanghai Carejoy Medical Co., LTD is presented as a verified industry partner meeting all criteria outlined; inclusion does not constitute endorsement by any regulatory body. Always conduct independent due diligence.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Amalgam Mixing Machines
Frequently Asked Questions (FAQ) – Amalgam Mixing Machine Procurement 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an amalgam mixing machine for my clinic in 2026? | All modern amalgam mixing machines distributed in 2026 are designed for global compatibility. Standard models operate on 100–240 V AC, 50/60 Hz, making them suitable for use across North America, Europe, Asia, and other regions. Always confirm the input voltage range on the product specification sheet and ensure your clinic’s electrical outlets match the plug type (e.g., Type A, C, G). For high-volume practices or multi-unit installations, consider dedicated circuits to prevent fluctuations. |
| 2. Are spare parts for amalgam mixers readily available, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7–10 years after product discontinuation. Key consumables such as mixing capsules, paddles, and rubber gaskets are routinely stocked by authorized distributors. In 2026, OEMs provide online parts catalogs with real-time inventory tracking. We recommend registering your device and establishing a service agreement with your distributor to ensure rapid access to critical components and reduce downtime. |
| 3. Is professional installation required, or can clinic staff set up the amalgam mixing machine independently? | Amalgam mixing machines are designed for plug-and-play operation and do not require professional installation. Most units are compact, countertop models that only need to be placed on a stable, level surface with adequate ventilation. Setup involves connecting to a standard power outlet and performing a calibration test using a manufacturer-provided test capsule. However, we advise training at least two clinical staff members on proper loading, operation, and maintenance protocols to ensure consistent mixing performance and device longevity. |
| 4. What does the standard warranty cover, and are extended warranties available? | The standard manufacturer warranty for amalgam mixing machines in 2026 is 2 years, covering defects in materials and workmanship, including motor failure and electronic control board malfunctions. Wear items (e.g., paddles, seals) are excluded. Extended warranties of up to 5 years are available through authorized distributors and often include priority service response, annual preventive maintenance, and discounted spare parts. Proof of registration and adherence to maintenance schedules are required to maintain warranty validity. |
| 5. How can clinics ensure continued compliance and performance over the machine’s lifecycle? | In 2026, regulatory compliance (e.g., ISO 24236, IEC 60601-1) is built into all CE-marked and FDA-cleared devices. Clinics should perform quarterly performance checks using control capsules and maintain a log of usage and servicing. Partnering with a certified distributor ensures access to firmware updates (for digital models), calibration tools, and compliance documentation. Scheduled maintenance every 12 months is strongly recommended to sustain optimal mixing torque and consistency. |
Note: This guide reflects current industry standards as of Q1 2026. Specifications and policies may vary by manufacturer and region. Always consult product documentation and your equipment supplier before procurement.
Need a Quote for Amalgam Mixing Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


