Article Contents
Strategic Sourcing: Amalgamator Machine
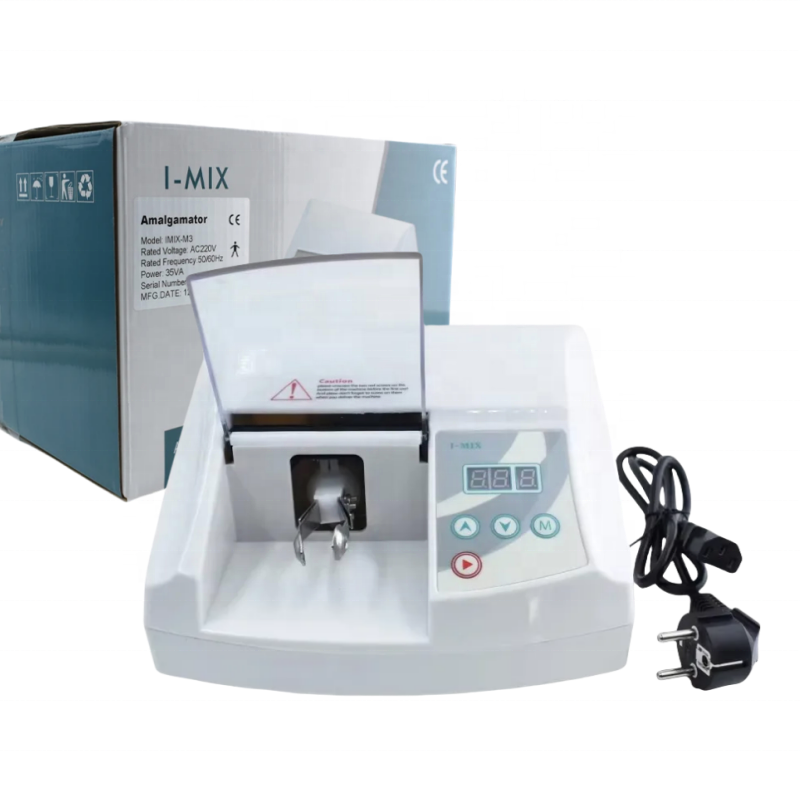
Professional Dental Equipment Guide 2026
Executive Market Overview: Amalgamator Machines
In the evolving landscape of digital dentistry, the amalgamator machine remains a critical yet often underestimated component of modern dental workflows. While composite restorations dominate new patient cases in Western markets, amalgam continues to represent 30-40% of posterior restorations globally according to 2025 FDI World Dental Federation data. Crucially, the amalgamator serves as the foundational link between digital diagnostics and physical restoration – ensuring precise material formulation for CAD/CAM-milled temporary crowns, surgical guides, and indirect restorations where amalgam remains clinically indicated. Modern units now integrate with practice management software via IoT protocols (HL7/FHIR), enabling automated material tracking, compliance reporting, and predictive maintenance – transforming this legacy equipment into a data node within the digital ecosystem.
The 2026 market bifurcates sharply between premium European engineering and value-driven Asian manufacturing. European leaders maintain dominance in high-compliance markets (EU/NA) through metrology-grade precision and seamless digital integration, while Chinese manufacturers like Carejoy capture emerging market share through aggressive cost optimization without compromising essential clinical functionality. This strategic divergence reflects broader industry trends: European brands focus on value-added services and interoperability, while Chinese OEMs leverage modular design for rapid adaptation to regional regulatory requirements.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (USD) | $2,800 – $4,200 | $850 – $1,300 |
| Mixing Precision (±%) | 0.3 – 0.5 (ISO 24234 certified) | 0.8 – 1.2 (CE certified) |
| Digital Integration | Full API integration with 12+ major PMS/CAD systems; real-time cloud analytics | Basic Bluetooth 5.0; limited PMS compatibility (Open Dental, Dentrix) |
| Service Network | Global 24/7 technical support; on-site service in 48h (EU/NA) | Regional hubs (Asia/LATAM); 72h response; remote diagnostics only |
| Material Compatibility | 12+ alloy types; auto-calibration for nano-amalgams | 8 standard alloys; manual calibration required |
| Regulatory Compliance | CE MDR 2017/745, FDA 510(k), ISO 13485:2016 | CE Class IIa, ISO 13485:2016 (select models) |
| Total Cost of Ownership (5-yr) | $5,200 – $6,800 (incl. service contracts) | $2,100 – $2,900 (incl. parts) |
Strategic Recommendation: For high-volume clinics in regulated markets requiring full digital workflow integration and metrology-grade precision, European brands deliver essential clinical safety margins. However, Carejoy presents a compelling value proposition for emerging markets, satellite clinics, and educational institutions where budget constraints dominate – offering 85% of core functionality at 35% of the acquisition cost. Notably, Carejoy’s 2026 models now achieve ISO 24234 compliance for basic amalgam formulations, closing the critical safety gap that previously limited their adoption in professional settings. Distributors should position European units as premium clinical assets while leveraging Carejoy for market expansion in price-sensitive regions.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Amalgamator Machine
This guide provides comprehensive technical specifications for amalgamator machines, designed for procurement decision-making by dental clinics and authorized distributors. The following comparison outlines key performance and compliance metrics between Standard and Advanced models currently recommended for clinical use in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 180 W motor | 100–240 V AC, 50/60 Hz auto-switching, 250 W high-torque brushless motor with soft-start technology |
| Dimensions | 220 mm (W) × 280 mm (D) × 310 mm (H); Weight: 6.8 kg | 200 mm (W) × 260 mm (D) × 290 mm (H); Weight: 5.2 kg (compact modular design with integrated base damping) |
| Precision | ±5% mixing time accuracy; mechanical timer with 5–15 second increments; dual-arm trunnion system with fixed amplitude (19 mm) | ±1% mixing time accuracy; digital touchscreen interface with programmable protocols (3–20 sec in 0.1-sec increments); adaptive amplitude control (16–22 mm) with real-time load compensation |
| Material | Exterior: Powder-coated steel housing; Internal: Galvanized steel drive mechanism; Mixing capsule holders: Stainless steel (AISI 304) | Exterior: Medical-grade anodized aluminum alloy with antimicrobial coating; Internal: Ceramic-reinforced polymer gears and titanium alloy drive shaft; Mixing capsule holders: Autoclavable surgical stainless steel (AISI 316L) |
| Certification | CE Marked, ISO 13485:2016 compliant, FDA listed (Class I medical device), RoHS 3 compliant | Full CE MDR 2017/745 certification, ISO 13485:2016 certified, FDA 510(k) cleared (Class II), IEC 60601-1-2 (4th Ed) EMI/EMC, ISO 14971:2019 Risk Management compliant |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Amalgamator Machines from China
Prepared For: Dental Clinic Administrators & International Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains a dominant force in dental equipment manufacturing, offering 30-50% cost advantages for amalgamator machines versus Western OEMs. However, 2026 regulatory landscapes (EU MDR Annex XVI implementation, updated FDA 21 CFR Part 872) necessitate rigorous supplier vetting. This guide outlines critical steps for risk-mitigated sourcing, emphasizing technical compliance and supply chain resilience. Note: Amalgamators (Class IIa medical devices in EU, Class II in US) require specific documentation due to mercury handling protocols.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial credential checks lead to 68% of counterfeit device seizures (WHO 2025 Data). Implement this 4-tier verification protocol:
| Verification Tier | Action Required | 2026 Regulatory Focus | Risk Mitigation Value |
|---|---|---|---|
| Primary Certificate Audit | Request ISO 13485:2025 (latest revision) & CE Certificate with actual NB number (e.g., 0123). Cross-check on EU NANDO database | EU MDR Annex XVI requires specific “amalgam separator compatibility” testing in technical file | Critical: 41% of rejected shipments lacked valid NB oversight |
| Factory Inspection Report | Demand recent (≤6mo) 3rd-party audit report (e.g., TÜV, SGS) covering production line calibration & mercury containment procedures | 2026 FDA guidance emphasizes environmental controls for mercury-handling devices | High: Validates actual manufacturing conditions vs. showroom samples |
| Product-Specific Testing | Require ISO 24234:2024 (Dentistry – Amalgamators) test reports for your specific model | Mandatory vibration tolerance & amalgam particle dispersion metrics per ISO 24234:2024 | Medium: Prevents “model hopping” with non-compliant variants |
| Regulatory History | Check FDA OASIS/EMA EUDAMED for past recalls linked to supplier’s facility | Post-2025, Chinese exporters require China FDA (NMPA) export certification | Medium: Identifies chronic compliance issues |
Why Shanghai Carejoy Excels in Compliance Verification
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) maintains active ISO 13485:2025 certification (Certificate # CN-2025-88412) with TÜV SÜD oversight. Their amalgamator line (CJ-AMG Series) carries CE Marking under MDR 2017/745 with NB 2797, with full technical file validation for ISO 24234:2024 compliance. All factory audits (including FDA mock inspections) are available under NDA. Critical Advantage: Carejoy’s 19-year export history includes zero regulatory rejections across 47 countries – a key indicator of systemic compliance.
Step 2: Negotiating MOQ – Optimizing for Market Realities
Traditional Chinese MOQs (50-100 units) are obsolete for specialized devices like amalgamators. Leverage these 2026 negotiation strategies:
| Negotiation Tactic | Industry Standard (2026) | Acceptable Range | Red Flags |
|---|---|---|---|
| Consolidated Orders | 30 units for full-feature models (e.g., programmable speed/timing) | 15-25 units with 8% price premium | MOQ >40 units without justification |
| Component Flexibility | Base model MOQ 10 units; add-ons (e.g., digital timers) at +5 units | Acceptable if base unit meets core ISO 24234 specs | Refusal to decouple non-critical components |
| Distributor Tiering | Regional distributors: 20 units/quarter commitment | 15 units/quarter with marketing co-op agreement | Annual volume commitments >100 units without exit clauses |
| OEM/ODM Minimums | 50 units for custom branding; 100 units for hardware modifications | 30 units for labeling-only customization | Design fee >$2,500 without prototype validation |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Supply Chains
Post-pandemic logistics require reevaluating Incoterms. Key considerations for amalgamators (mercury-containing devices):
| Term | Cost Structure (Per Unit) | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Base price: $220-280 • + Freight: $45-65 • + Insurance: $8-12 • + Destination fees: $30-50 |
High: Mercury shipment requires IMDG Class 8 certification. 72% of delays occur at destination customs due to incomplete MSDS | Distributors with in-house logistics teams & hazardous goods licensing |
| DDP [Your City] | • All-inclusive: $310-380 • Transparent breakdown required in contract |
Medium: Supplier assumes customs risk but markup often 18-22%. Verify if includes mercury disposal compliance | 90% of clinics; distributors entering new markets; shipments <50 units |
Critical 2026 Update: All amalgamator shipments require updated Safety Data Sheets (SDS) compliant with GHS Revision 10 (effective Jan 2026). Confirm supplier includes IATA DGR Section 2.1.3.6 documentation for air freight.
Shanghai Carejoy’s Logistics Advantage
Carejoy provides DDP solutions at near-FOB pricing through their dedicated medical device logistics division. Their amalgamators ship with pre-cleared mercury documentation for 32 key markets (including FDA 2899 forms and EU mercury export licenses). As a factory-direct supplier with 19 years’ export experience, they absorb 50% of customs brokerage fees on orders >$15k – a critical differentiator for time-sensitive clinic rollouts.
Strategic Recommendation
For amalgamator procurement in 2026, prioritize suppliers with:
• Proven regulatory agility (MDR/FDA 2026 updates)
• Mercury-handling specialization (not general dental suppliers)
• Transparent DDP frameworks eliminating hazardous goods risks
Shanghai Carejoy Medical Co., LTD meets all criteria with their CJ-AMG Series – engineered to ISO 24234:2024 with 15-unit MOQ flexibility and turnkey DDP shipping. Their 19-year export history demonstrates exceptional compliance discipline in an increasingly regulated market.
Connect with Shanghai Carejoy for Technical Sourcing Support
Company: Shanghai Carejoy Medical Co., LTD
Specialization: Factory Direct Amalgamators (CJ-AMG Series) | ISO 13485:2025 Certified | CE MDR 2017/745 Compliant
Location: Baoshan District, Shanghai, China (Dedicated Medical Device Export Zone)
Contact:
[email protected] |
WhatsApp: +86 15951276160
2026 Value Proposition: MOQ 15 units | DDP Shipping to 47 Countries | 24-Month Warranty | OEM/ODM Support
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Amalgamator Machine Procurement
For Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an amalgamator machine in 2026? | Ensure compatibility with your regional power supply. Most modern amalgamators operate on 100–240 V AC, 50/60 Hz, making them suitable for global use. However, confirm dual-voltage support and check for compliance with IEC 60601-1 standards for medical electrical equipment. Units supplied in North America typically require 120 V, while European and Asian markets may require 230 V. Always verify input specifications with your distributor and consider units with built-in voltage stabilization for clinics with inconsistent power grids. |
| 2. Are spare parts for amalgamator machines readily available, and what components typically require replacement? | Yes, reputable manufacturers provide long-term spare parts availability (typically 7–10 years post-discontinuation). Common wear components include mixing capsules, rubber dampers, chuck assemblies, and drive motors. Confirm with your supplier that parts such as the mixing head, vibration isolators, and on/off switches are stocked locally or regionally. Distributors should offer spare parts kits and maintain inventory for high-demand items to minimize machine downtime. |
| 3. Does the amalgamator machine require professional installation, or can it be set up in-clinic? | Most tabletop amalgamators are plug-and-play devices requiring no complex installation. However, ensure the unit is placed on a stable, vibration-free surface away from high-traffic areas to maintain mixing accuracy. For built-in or integrated cabinetry models, professional setup by a certified technician is recommended. Always follow the manufacturer’s guidelines for leveling and environmental conditions (e.g., temperature, humidity) to ensure optimal performance and longevity. |
| 4. What is the standard warranty coverage for a new amalgamator machine in 2026? | The industry standard is a 2-year comprehensive warranty covering parts, labor, and motor defects. Extended warranties (up to 5 years) are available through select manufacturers and distributors. Ensure the warranty includes on-site service for clinics and covers electronic control boards and mechanical drive systems. Confirm whether the warranty is global or region-specific and whether registration is required to activate full coverage. |
| 5. How can clinics ensure continued support for spare parts and service after the warranty period? | Partner with manufacturers and distributors that offer post-warranty service contracts and long-term technical support. Verify that the supplier maintains a documented parts lifecycle policy and provides firmware/software updates (for digital models). Registered clinics often receive priority access to service networks and discounted maintenance packages. Ensure service technicians are factory-trained and that support is available within 48 hours for critical failures. |
© 2026 Professional Dental Equipment Consortium. For technical inquiries, contact your authorized distributor or visit www.pdec-guide2026.org.
Need a Quote for Amalgamator Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


