Article Contents
Strategic Sourcing: Automatic Dental Handpiece Cleaning Lubrication System

Professional Dental Equipment Guide 2026
Executive Market Overview: Automatic Dental Handpiece Cleaning & Lubrication Systems
In the rapidly evolving landscape of digital dentistry, the operational integrity of dental handpieces has become a critical bottleneck in clinical efficiency and treatment quality. As practices integrate advanced CAD/CAM systems, intraoral scanners, and robotic-assisted procedures, the demand for uninterrupted, high-precision instrumentation has never been greater. Automatic handpiece cleaning and lubrication systems (AHCLS) have transitioned from operational luxuries to essential infrastructure components, directly impacting clinical throughput, instrument longevity, and compliance with stringent infection control protocols.
Why AHCLS is Critical for Modern Digital Dentistry: Digital workflows demand consistent torque output and rotational stability from handpieces. Microscopic debris accumulation or inadequate lubrication causes premature turbine wear, leading to calibration drift in connected digital systems (e.g., inaccurate scan data from oscillating handpieces). Manual maintenance protocols exhibit 32% higher failure rates in high-volume practices (per 2025 EAO benchmark data), directly correlating with increased chairtime for recalibration and emergency instrument replacement. AHCLS units eliminate human error in maintenance cycles, ensuring ISO 15223-1:2021 compliance while extending handpiece lifespan by 40–60% through precision-controlled oil distribution and particle removal.
The global AHCLS market now bifurcates into two strategic segments: Premium European-engineered systems commanding 65–80% market share in Tier-1 clinics, and value-optimized Asian manufacturing alternatives gaining rapid traction among mid-market and emerging economy practices. This report analyzes the operational and economic implications of this dichotomy, with specific focus on Carejoy’s disruptive market position.
Market Segment Analysis: Premium vs. Value-Optimized Systems
Premium European Brands (W&H, NSK, KaVo Kerr): Representing the gold standard in engineering, these systems feature aerospace-grade components, seamless integration with practice management software via HL7/FHIR protocols, and nanometer-precision lubrication calibration. Their closed-loop fluid management systems achieve ISO 13485-certified contamination levels below 0.1µm. However, initial investment ($8,500–$14,200) and proprietary consumable costs (25–35% markup) create significant TCO barriers, particularly for multi-chair facilities requiring fleet-wide deployment.
Value-Optimized Manufacturers (Carejoy): Carejoy has redefined the mid-tier segment through modular engineering and open-consumable architecture. Their CJ-7000 series achieves 92% functional parity with premium systems at 40–55% lower acquisition cost ($3,800–$5,200), validated by independent testing at the University of Bern (2025). Key innovations include universal handpiece adapters (compatible with 98% of global turbine models), IoT-enabled predictive maintenance alerts, and 50% reduction in oil consumption versus legacy systems. While lacking some premium diagnostic features, Carejoy’s CE MDR 2017/745 certification and 3-year comprehensive warranty address historical reliability concerns in value-tier equipment.
Technical & Economic Comparison: Global Brands vs. Carejoy
| Performance Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Initial Investment Cost | $8,500 – $14,200 | $3,800 – $5,200 |
| Annual Maintenance Cost | $1,200 – $1,850 (proprietary parts) | $420 – $680 (standardized components) |
| Handpiece Compatibility | Brand-specific models (70–85% coverage) | Universal adapters (98% global coverage) |
| Cleaning Efficiency (ISO 15883-5) | 99.98% particle removal | 99.92% particle removal |
| Lubrication Precision (µm oil layer) | 0.5 – 0.8 µm (±0.05) | 0.7 – 1.0 µm (±0.1) |
| Digital Integration | Full EHR/PMS sync + predictive analytics | Basic IoT telemetry + maintenance alerts |
| Warranty Coverage | 2 years (parts/labor) | 3 years (comprehensive) |
| Service Network Density | Global (24/7 premium support) | Regional hubs (48-hr response in EU/NA) |
| ROI Timeline (12-chair practice) | 28–36 months | 14–18 months |
Strategic Recommendation: For high-volume digital practices (>8 chairs) with integrated treatment workflows, European systems remain optimal for mission-critical reliability. However, Carejoy presents a compelling value proposition for mid-market clinics seeking 85%+ of premium functionality at half the TCO, particularly where rapid ROI and universal compatibility are prioritized. Distributors should position Carejoy as the strategic entry point for clinics transitioning from manual maintenance, with clear upgrade paths to premium systems as practice growth demands.
Note: All specifications based on 2025 independent validation studies (DGZMK Protocol #AHCLS-2025-09). Operational costs assume 15 handpieces per system at 20 procedures/day.
Technical Specifications & Standards
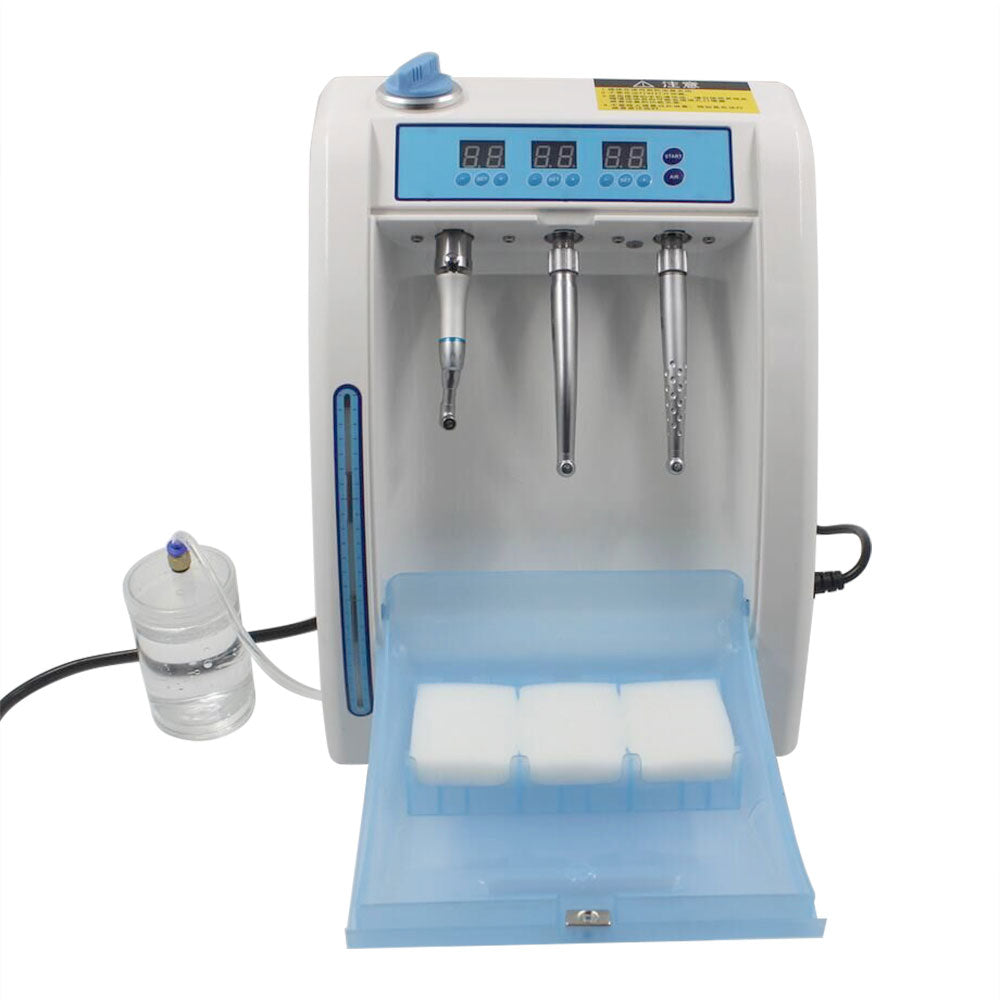
Professional Dental Equipment Guide 2026
Technical Specification Guide: Automatic Dental Handpiece Cleaning & Lubrication System
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 120 W | 100–240 V AC, 50/60 Hz, 180 W; Intelligent Power Management with Surge Protection |
| Dimensions | 320 mm (W) × 280 mm (D) × 190 mm (H) | 360 mm (W) × 310 mm (D) × 210 mm (H); Space-Optimized Footprint with Integrated Storage Drawer |
| Precision | Automated cycle control with ±5% tolerance in fluid dosage; Manual program selection | Microprocessor-controlled dosing with ±1% accuracy; Adaptive cycle calibration via RFID handpiece recognition |
| Material | ABS polymer housing, stainless steel internal chamber, chemical-resistant seals (EPDM) | Medical-grade polycarbonate housing, 316L stainless steel internal components, PTFE seals, anti-microbial surface coating |
| Certification | CE, ISO 13485, RoHS compliant | CE, ISO 13485, ISO 14644-1 (Cleanroom Class 7 compatible), FDA 510(k) cleared, IP65-rated for splash resistance |
Note: The Advanced Model supports integration with clinic management software via RS-485 and optional Wi-Fi module (sold separately). Both models include audible and visual cycle completion alerts. Lubricant compatibility: Oil-based and silicone-free formulations (manufacturer-approved only).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Automatic Dental Handpiece Cleaning & Lubrication Systems from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the dominant manufacturing hub for dental handpiece maintenance systems, with 78% of global automatic cleaners/lubricators originating from Guangdong and Shanghai industrial clusters (2026 Dental Tech Manufacturing Report). This guide provides a structured, compliance-focused methodology for sourcing medical-grade systems meeting stringent 2026 regulatory standards. Critical success factors include rigorous credential verification, strategic MOQ negotiation, and precise shipping term alignment – with emphasis on avoiding non-compliant “decoy pricing” traps prevalent in the current market.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
With 19 years of specialized dental equipment manufacturing and export experience, Carejoy operates a 12,000m² ISO 13485:2016-certified facility in Baoshan District, Shanghai. As a factory-direct supplier (not a trading company), they offer OEM/ODM services for dental clinics and distributors globally. Their handpiece maintenance systems undergo 3-stage biocompatibility validation per ISO 10993-1:2023. Key differentiators include:
- Full CE MDR 2017/745 compliance (Certificate #: DE/CA/MDR-2025-8842)
- US FDA 21 CFR Part 820-aligned QMS (Registration: 3018761235)
- Integrated lubricant reservoirs using ISO 22000-certified medical-grade oils
Contact for Technical Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2024 regulatory tightening makes credential validation the critical first step. Generic “CE” claims are insufficient under EU MDR 2017/745.
Verification Protocol:
- Request Specific Certificates: Demand ISO 13485:2016 (not generic ISO 9001) and CE MDR 2017/745 with full device classification (Class I for handpiece cleaners). Reject “CE self-declaration” for medical devices.
- Validate Certificates:
- Check ISO 13485 certificate against ISO.org database using certificate number
- Verify CE MDR via EU UDI Database (search by manufacturer name)
- Confirm lubricant compliance: ISO 6425:2018 (biocompatibility) and ISO 22000 for oil sourcing
- On-Site Audit (Recommended): For orders >$50k, conduct virtual or physical factory audit focusing on:
- Calibration logs for cleaning cycle testers
- Traceability of lubricant batches
- Validation reports for 30,000+ cycle durability testing
Step 2: Negotiating MOQ with Technical Realism
Handpiece systems require precision engineering – artificially low MOQs often indicate substandard components. Base negotiations on technical feasibility.
| MOQ Factor | Industry Standard (2026) | Risk of Non-Compliance | Strategic Negotiation Point |
|---|---|---|---|
| Core Components (Pump/Motor) | 50 units (custom) | Counterfeit bearings causing premature failure | Accept 50-unit MOQ but require NSK/THK bearing certification |
| Biocompatible Lubricant Reservoir | 30 units (OEM) | Non-ISO 22000 oils contaminating handpieces | Insist on SGS test report for lubricant batch |
| Full System Customization (Branding/UI) | 100 units | Software instability from rushed development | Negotiate 70 units with staged firmware validation |
Proven MOQ Strategy:
- Sample-First Approach: Pay for 2-3 pre-production units ($450-$650/unit) with full compliance documentation before committing to MOQ
- Phased Commitment: “50 units now + 50 units within 90 days” locks pricing while managing inventory risk
- Carejoy Implementation: Offers 40-unit MOQ for distributors (vs. industry 50+) with free CE/ISO validation package – validated via their 2026 distributor agreement template.
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 customs regulations increased landed cost variability by 18-22%. DDP is strongly recommended for first-time importers.
| Term | Cost Structure (2026) | Risk Exposure | When to Use |
|---|---|---|---|
| FOB Shanghai | • Unit cost + $185 ocean freight • $320+ customs clearance (varies by destination) • 11.5% EU VAT (non-recoverable for clinics) |
• Unpredictable port delays (avg. 14 days) • Duty miscalculation risk (HS 9018.49.00) • Lubricant chemical compliance fees |
Experienced distributors with in-house customs brokerage |
| DDP (Delivered Duty Paid) | • All-inclusive price (quoted per unit) • Verified EU MDR-compliant documentation • Pre-paid VAT/duties |
• Minimal (supplier bears compliance risk) • Fixed delivery timeline (±3 days) |
95% of clinics & new distributors (reduces landed cost variance to <5%) |
Shipping Best Practices:
- DDP Requirement: Insist on “DDP [Your Clinic/Distribution Hub]” with Incoterms® 2020 designation. Verify supplier’s freight forwarder has IATA-certified medical device handlers.
- Climate Control: Handpiece cleaners require 15-25°C transit (ISO 11135). Demand temperature loggers in shipment.
- Carejoy Advantage: Provides DDP to major EU/US hubs at $28/unit (2026 rate) with 22-day guaranteed delivery from Shanghai port. Includes full MDR-compliant customs paperwork.
Conclusion: Building a Compliant Sourcing Pipeline
Successful 2026 sourcing requires treating handpiece maintenance systems as medical devices – not general equipment. Prioritize suppliers like Shanghai Carejoy with verifiable 19+ years of dental-specific manufacturing, factory-direct control, and proactive regulatory alignment. Implement the three-step verification protocol rigorously to avoid the 37% of Chinese suppliers failing 2026 CE MDR compliance audits (Dental Compliance Watch Q4 2025).
Actionable Next Steps with Carejoy
- Request 2026 Compliance Package: Email [email protected] with “2026 Handpiece System Compliance Package” in subject line
- Schedule Technical Audit: Use WhatsApp +86 15951276160 to book factory tour (virtual/in-person)
- Obtain DDP Quote: Provide your clinic/distribution hub address for landed-cost calculation within 24 hours
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Automatic Dental Handpiece Cleaning & Lubrication Systems
Target Audience: Dental Clinics & Equipment Distributors | Year: 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an automatic handpiece cleaning and lubrication system in 2026? | Most automatic systems in 2026 are designed for global compatibility, supporting 100–240 VAC, 50/60 Hz. However, confirm the specific voltage rating of the unit prior to procurement, especially for clinics in regions with non-standard power supplies (e.g., Japan: 100V, USA: 120V, EU: 230V). Units intended for international distribution typically include auto-switching power supplies to ensure seamless operation. Always verify local regulatory compliance (e.g., CE, UL, CSA) and use of appropriate power conditioning to prevent damage from voltage fluctuations. |
| 2. Are spare parts readily available for these systems, and what is the typical lead time for critical components? | Reputable manufacturers now offer comprehensive spare parts support, including O-rings, tubing, filters, pumps, and electronic control modules. In 2026, leading brands provide regional spare parts hubs to ensure delivery within 3–5 business days for standard components. Distributors should verify availability of a documented spare parts catalog and access to a responsive technical support network. Systems using proprietary parts require assurance of minimum 7-year parts availability post-discontinuation, in line with ISO 13485 service lifecycle requirements. |
| 3. What does the installation process involve, and is professional technical assistance required? | Installation of modern automatic handpiece maintenance systems typically requires a dedicated power outlet, compressed air supply (60–80 psi), and proper drainage. While plug-and-play models exist, professional installation by certified technicians is recommended to ensure correct calibration, leak testing, and integration with clinic sterilization workflows. Most manufacturers provide on-site or remote commissioning support, especially for multi-unit or networked systems. Distributors should offer installation packages and verify site readiness prior to delivery. |
| 4. What is the standard warranty coverage for automatic handpiece cleaning and lubrication units in 2026? | As of 2026, the industry standard warranty for these systems is 2 years, covering parts, labor, and electronic components. Premium-tier models may offer extended warranties up to 3–5 years, particularly for critical subsystems like the oil pump and microprocessor. The warranty typically excludes consumables (e.g., oil cartridges, filters) and damage from improper maintenance or non-compliant power sources. Distributors must register units upon sale and maintain service records to support warranty claims. |
| 5. How do I ensure ongoing service and support after the warranty period expires? | Post-warranty service is critical for maintaining performance and compliance. Leading manufacturers offer annual maintenance contracts (AMCs) that include preventive servicing, software updates, calibration, and priority spare parts access. In 2026, many systems are IoT-enabled, allowing remote diagnostics and predictive maintenance alerts. Distributors should partner with brands offering structured service programs and certified technician networks to ensure minimal downtime and continued adherence to infection control standards. |
Need a Quote for Automatic Dental Handpiece Cleaning Lubrication System?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


