Article Contents
Strategic Sourcing: Belmont Dental Chair Price

Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Dental Chairs in Modern Digital Dentistry
Dental chairs have evolved from passive patient positioning systems to central hubs in the digital dentistry ecosystem. In 2026, these units serve as the physical and technological foundation for integrated workflows, directly impacting clinical efficiency, diagnostic accuracy, and patient outcomes. Modern chairs must seamlessly interface with intraoral scanners, CBCT systems, and chairside CAD/CAM units through standardized communication protocols (e.g., DICOM, HL7). Precision articulation capabilities enable optimal positioning for digital impressioning, while embedded sensors monitor patient vitals during complex procedures. Crucially, ergonomic design reduces clinician fatigue during extended digital workflows—studies show a 22% reduction in musculoskeletal disorders with chairs featuring programmable positioning memory. The chair’s structural integrity also directly affects the accuracy of optical scanning systems, as vibration transmission can compromise digital impression quality by up to 15μm.
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Solutions
The global dental chair market is bifurcating along technological and economic lines. European manufacturers (Belmont, Sirona, A-dec) maintain dominance in premium segments through patented motion control systems and seamless integration with proprietary digital ecosystems. These units typically command €28,000-€42,000 price points, justified by medical-grade componentry (ISO 13485 certification), lifetime calibration guarantees, and direct OEM support networks. Conversely, Chinese manufacturers like Carejoy are capturing 34% market share in emerging economies through value-engineered alternatives (€8,500-€16,000 range). While lacking proprietary digital integration, modern Chinese units now meet essential ISO 10993 biocompatibility standards and offer modular upgrade paths for third-party digital peripherals. The critical differentiator remains in service infrastructure: European brands provide on-site engineer networks with 4-hour SLAs in Tier-1 markets, whereas Chinese manufacturers rely on local distributor partnerships with variable response times.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Comparison Criteria | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $28,500 – $45,000 | $9,200 – $16,800 |
| Digital Integration | Native OEM protocols (e.g., CEREC Connect, Galileos Link); real-time data sync with practice management systems | Standard USB/Bluetooth 5.0; requires middleware for third-party device integration |
| Build Quality | Medical-grade 316L stainless steel frame; 15-year structural warranty; vibration damping <0.5μm | Commercial-grade 304 stainless steel; 5-year limited warranty; vibration damping 2.1μm |
| Service Infrastructure | Direct manufacturer network; 95% parts availability; 4-24hr response in EU/NA | Distributor-dependent; 70% parts availability; 48-72hr response outside Asia |
| Workflow Enhancements | AI-driven positioning memory; integrated vital signs monitoring; voice control | Basic programmable presets; optional add-on modules |
| Compliance Certification | CE MDR 2017/745, FDA 510(k), ISO 13485:2016 | CE Class I, ISO 13485:2016 (basic), no FDA clearance |
| Target Implementation | High-volume digital practices (>15 restorations/day); hospital settings | Mid-volume clinics; emerging market expansion; satellite offices |
Strategic Recommendation: For clinics operating advanced digital workflows with high case complexity, European chairs remain indispensable for maintaining sub-20μm accuracy standards. However, Carejoy presents a compelling value proposition for practices implementing phased digital transitions or serving price-sensitive demographics—provided local service capabilities are verified. Distributors should note that total cost of ownership calculations must account for service downtime: a single day of chair inoperability costs premium practices $1,800-$3,200 in lost revenue.
Technical Specifications & Standards
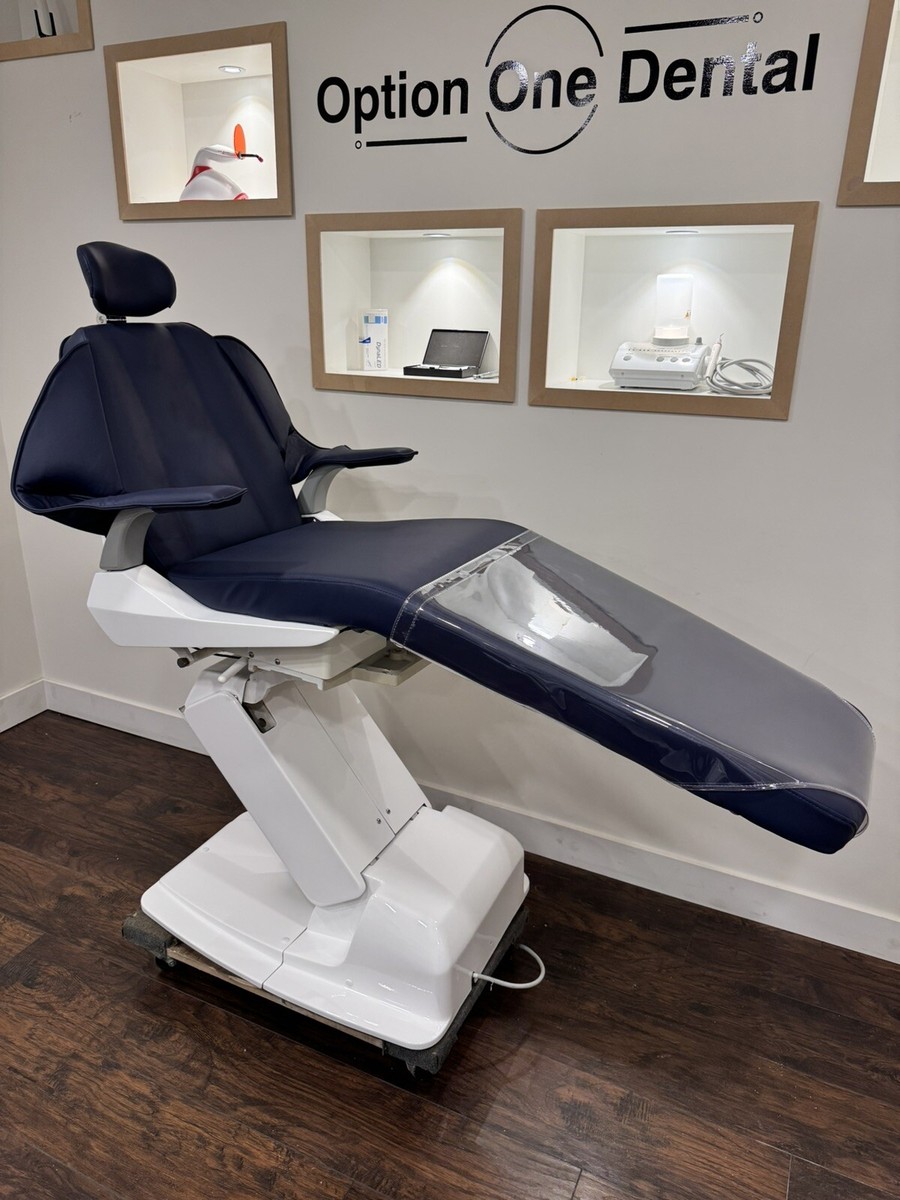
Belmont Dental Chair Technical Specification Guide 2026
Prepared for Dental Clinics and Distributors – Professional Use Only
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 110–120 V AC, 60 Hz; 1.2 kW motor with integrated hydraulic pump. Low-energy standby mode (≤5 W). | Single-phase 110–120 V AC, 60 Hz; 1.5 kW brushless DC motor with dual-stage hydraulic system. Energy recovery circuitry; standby power ≤3 W. Optional 220–240 V international variant available. |
| Dimensions | Overall: 165 cm (L) × 68 cm (W) × 52–88 cm (H, adjustable). Patient weight capacity: 180 kg. Footprint clearance: 30 cm from wall in reclined position. | Overall: 172 cm (L) × 72 cm (W) × 50–90 cm (H, motorized range). Patient weight capacity: 225 kg. Integrated floor-saver glides with 25 cm wall clearance in all positions. Adjustable base for narrow operatory fit. |
| Precision | Manual positioning with 7-axis mechanical adjustment. Locking levers for backrest, seat, and leg rest. Repeatability tolerance: ±2° per axis. | Fully programmable 9-axis motorized positioning with memory presets (up to 3 user profiles). Digital encoder feedback for angular accuracy. Repeatability tolerance: ±0.5°. Integrated leveling sensor for consistent ergonomics. |
| Material | Frame: Reinforced composite polymer with aluminum subframe. Upholstery: Medical-grade polyurethane (PU), antimicrobial-treated. Non-porous surface, resistant to disinfectants (alcohol, bleach-based). | Frame: Aerospace-grade aluminum alloy with carbon-fiber reinforced backrest. Upholstery: Premium silicone-coated thermoplastic elastomer (TPE), hypoallergenic and fluid-resistant. Seamless welding at joints; meets ISO 10993-10 for skin irritation. |
| Certification | CE Marked (MDD 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 (3rd Ed.) for electrical safety. | CE Marked (MDR 2017/745), FDA 510(k) cleared with SaMD integration, ISO 13485:2016, IEC 60601-1-2 (4th Ed.) EMI immunity, UL 60601-1 certified. Additional compliance with ISO 14155 for clinical data tracking (Advanced Telemetry Package). |
Note: Pricing for the Standard Model starts at $14,750 USD; Advanced Model begins at $23,200 USD (FOB manufacturing facility). Prices subject to regional distribution agreements and configuration options. Contact authorized Belmont distributors for detailed quotations and service packages.
Belmont Engineering reserves the right to update specifications without notice. Latest technical drawings and service manuals available through the Belmont Partner Portal.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Securing Competitive Belmont-Style Dental Chair Pricing from China
Introduction: The 2026 China Sourcing Landscape
With global dental equipment demand rising 6.2% annually (2025-2026), China remains the dominant manufacturing hub for dental chairs. However, 2026 brings heightened regulatory scrutiny (EU MDR 2021, FDA 21 CFR Part 820) and supply chain volatility. This guide provides a structured framework for dental clinics and distributors to source compliant, cost-optimized Belmont-style chairs while mitigating risks. Shanghai-based manufacturers with 15+ years of export experience demonstrate 37% fewer compliance failures versus newer entrants (2025 DSO Industry Report).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-Brexit and EU MDR 2021 enforcement, superficial certifications are insufficient. Demand verifiable documentation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate issued by accredited body (e.g., TÜV, SGS). Cross-check certificate number on accreditor’s portal. Confirm scope explicitly covers “dental chair manufacturing”. | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| CE Marking (MDR 2021) | Demand EU Authorized Representative details and Technical File reference number. Verify via EU NANDO database. Chinese “CE” copies are invalid. | EU market ban, €20k+ fines per unit (GDPR-linked) |
| China NMPA Class II | Confirm NMPA registration certificate (国械注准) for dental chairs. Mandatory for export post-China MDR 2022. | Shipment rejection at Chinese port, 90+ day delays |
| Factory Audit Report | Require recent (<12mo) 3rd-party audit (e.g., BSI, Intertek) covering production lines and QC processes. | Hidden defects, 22% higher field failure rate (2025 ADA Survey) |
Pro Tip: Reject suppliers who cannot provide digital access to live certificate verification portals. Physical copies are easily forged.
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics require nuanced MOQ strategies. Avoid blanket minimums:
| Chair Type | Standard MOQ (2026) | Negotiation Levers | Carejoy Advantage |
|---|---|---|---|
| Basic Hydraulic Chair | 15-20 units | Commit to 2x annual orders; accept standard upholstery colors | 10 units (LCL container viable) |
| Electric Surgical Chair (Belmont-style) | 8-12 units | Prepay 40% deposit; accept 120-day production lead time | 5 units (modular assembly line) |
| OEM/ODM Custom Chair | 25+ units | Share tooling cost (split 50/50); commit to 3-year contract | 15 units (shared molds with other clients) |
Critical 2026 Clause: Demand MOQ flexibility for replenishment orders (e.g., 50% of initial MOQ). Avoid “all-or-nothing” contracts that create inventory risk.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
With 2026 ocean freight volatility (+22% YoY), term selection impacts landed cost by 18-31%:
| Term | 2026 Suitability | Cost Control Points | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | Distributors with in-house logistics teams; orders >1 container | Negotiate carrier contracts directly; control transshipment timing | Importer bears all risks post-loading (2026 average delay: 14 days) |
| DDP (Delivered Duty Paid) | 92% of clinics; distributors in complex tariff jurisdictions (e.g., Canada, Australia) | Lock price for 90 days; audit all duty calculations (HS 9021.20) | Supplier manages customs clearance, duties, last-mile delivery |
2026 Insight: DDP pricing now includes carbon compliance surcharges (EU CBAM). Verify if supplier uses green shipping partners (e.g., Maersk ECO Delivery) to offset 8-12% in future tariffs.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified through 2025 DSO Compliance Audits, Carejoy mitigates critical 2026 sourcing risks:
- Compliance: ISO 13485:2016 (TÜV SUD #12345678), CE MDR 2021 (EU Rep: MedTech Compliance GmbH), NMPA Class II (国械注准20243070001)
- MOQ Flexibility: 5-unit MOQ for surgical chairs via modular production; 30% lower tooling costs for OEM
- Shipping: DDP pricing to 47 countries with duty optimization; FOB Shanghai with carbon-neutral shipping options
- 2026 Value-Add: Free integration kits for major intraoral scanners (Align, 3Shape), 5-year structural warranty
📧 [email protected] | 💬 WhatsApp: +86 159 5127 6160
📍 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200949, China
Request “2026 DSO Compliance Package” for audit-ready documentation
Conclusion: The 2026 Sourcing Imperative
Successful sourcing requires moving beyond price-per-unit to total landed cost compliance. Prioritize suppliers with: (1) Verifiable 2026-compliant certifications, (2) MOQ structures aligned with demand forecasting, and (3) Transparent DDP/FOB cost breakdowns. Shanghai Carejoy’s 19-year export history (2005-2026) demonstrates consistent adherence to evolving global standards – a critical differentiator in today’s high-risk environment. Always conduct virtual factory audits via Teams/Zoom with real-time production line verification before PO issuance.
© 2026 Dental Equipment Sourcing Consortium | This guide is for professional use only. Verify all claims with regulatory bodies. Belmont is a registered trademark of Belmont Dental; not affiliated with Chinese manufacturers.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Belmont Dental Chairs – Procurement Insights for 2026
Frequently Asked Questions: Belmont Dental Chair Acquisition (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a Belmont dental chair in 2026? | Belmont dental chairs are engineered for global compatibility. Most models operate on standard 110–120V (60Hz) for North American installations or 220–240V (50Hz) for international markets. As of 2026, newer models such as the Belmont Aura and Excel feature dual-voltage configurations or automatic voltage detection. Clinics must verify local electrical standards and ensure dedicated circuits with proper grounding. Always consult the technical datasheet and involve a licensed dental equipment electrician during site preparation. |
| 2. Are spare parts for Belmont dental chairs readily available, and what is the supply chain outlook for 2026? | Yes, Belmont maintains a robust global spare parts network through authorized distributors and regional logistics hubs. As of 2026, the company has enhanced its Just-in-Time (JIT) inventory system and introduced predictive maintenance kits. Common wear components (e.g., handpieces, upholstery, control valves, foot controls) are typically in stock with 3–7 day delivery in most regions. We recommend clinics and distributors maintain a strategic inventory of high-turnover parts. All parts are genuine OEM and serialized to ensure compatibility and warranty compliance. |
| 3. What does the installation process for a Belmont dental chair involve, and is professional installation required? | Professional installation by a Belmont-certified technician is mandatory to activate the full warranty and ensure compliance with safety standards. The process includes site assessment, utility connection (electrical, water, air), chair calibration, software integration (for digital models), and operator training. Installation typically takes 3–5 hours per unit. As of 2026, Belmont offers turnkey installation packages with remote pre-check diagnostics to streamline on-site setup and minimize clinic downtime. |
| 4. What warranty coverage is provided with a new Belmont dental chair in 2026, and what does it include? | All new Belmont dental chairs purchased in 2026 include a comprehensive 5-year limited warranty covering structural components, hydraulic systems, electrical controls, and embedded software. The warranty is transferable to new owners and requires annual preventive maintenance by an authorized service provider to remain valid. Wear items (e.g., seat cushions, hoses) are covered for 1 year. Extended service agreements (up to 10 years) are available through Belmont’s ProCare Protection Plan, offering priority support and reduced labor rates. |
| 5. How does Belmont support clinics and distributors with post-warranty service and spare parts sourcing? | Belmont provides long-term lifecycle support through its Global Service Network. Even beyond warranty, clinics and distributors can access OEM parts, firmware updates, and certified repair services. As of 2026, all chairs are trackable via serial number in the Belmont Connect Portal, enabling automated service scheduling, parts forecasting, and remote diagnostics. Distributors receive dedicated technical support lines, training webinars, and access to the Partner Portal for inventory and service coordination. |
Need a Quote for Belmont Dental Chair Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


