Article Contents
Strategic Sourcing: Belmont X Ray Machine

Executive Market Overview: Belmont X-ray Systems in the 2026 Digital Dentistry Landscape
Critical Role in Modern Digital Dentistry
Digital radiography has evolved from a diagnostic adjunct to the cornerstone of evidence-based dental treatment planning. The Belmont X-ray platform (now integrated within Dentsply Sirona’s ecosystem) represents the pinnacle of precision engineering for intraoral and panoramic imaging, delivering sub-micron resolution with dose reductions of up to 80% compared to legacy film systems. In 2026, these systems are non-negotiable for clinics pursuing ISO 13485 compliance and implementing AI-driven diagnostics, as they provide the foundational DICOM 3.0-compliant data stream required for seamless integration with CAD/CAM workflows, teledentistry platforms, and predictive analytics engines. Crucially, Belmont’s real-time dose monitoring and automatic exposure control (AEC) technology mitigate regulatory risks under tightening EU MDR 2023 radiation safety mandates.
Market Dichotomy: Premium Global Brands vs. Value-Oriented Manufacturers
The European dental imaging market is bifurcated between established premium brands (predominantly German, Swiss, and Finnish) and rapidly advancing Chinese manufacturers. Global leaders like Belmont (US heritage, European manufacturing), Planmeca, and Vatech command 65-75% market share in Tier-1 clinics through engineering excellence, but carry 40-60% higher TCO due to proprietary service ecosystems. Conversely, Chinese innovators like Carejoy are capturing 28% market growth in emerging EU economies by leveraging semiconductor cost advantages and modular architecture. While Carejoy systems meet essential CE MDR requirements, they lack the clinical validation depth and service infrastructure required for high-volume specialist practices. Distributors must weigh clinical risk tolerance against ROI timelines when advising clients.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Belmont, Planmeca, Vatech) |
Carejoy Systems |
|---|---|---|
| Price Range (Panoramic/CBCT) | €38,500 – €72,000 | €14,200 – €26,800 |
| Image Sensor Technology | CCD/CMOS with 16-bit depth, 4.5 lp/mm resolution | CMOS with 12-bit depth, 3.2 lp/mm resolution |
| Dose Management | AI-guided AEC, real-time dosimetry, <0.5 μSv/image | Fixed exposure presets, basic dosimetry, 1.2-1.8 μSv/image |
| Integration Ecosystem | Native DICOM 3.0, HL7, and direct PMS integration (Dentrix, Open Dental) | Proprietary SDK required for PMS integration; limited EDR compatibility |
| Service Infrastructure | 24/7 onsite support (EU-wide), 2-hour SLA, certified biomedical engineers | Regional hubs (DE/PL only), 48-hour SLA, third-party technicians |
| Advanced Diagnostics | Integrated AI caries detection (FDA 510k cleared), 3D cephalometric analysis | Basic 2D measurement tools; no AI diagnostics |
| Warranty & Lifecycle | 36-month comprehensive coverage, 10+ year parts availability | 12-month limited warranty, 5-year parts commitment |
| Ideal Clinical Application | Multi-specialty clinics, university hospitals, high-volume practices (>8k patients/year) | Single-operator clinics, emerging markets, satellite offices |
Technical Specifications & Standards

| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 8 mA tube current, 60 Hz AC input, 110–120 V ±10% power supply. Integrated high-frequency generator with automatic exposure control (AEC) for consistent image quality. | 90 kVp maximum output, 15 mA tube current, wide-range 100–240 V ±10%, 50/60 Hz auto-switching power supply. Advanced high-frequency inverter technology with dual-layer AEC and real-time dose optimization algorithms. |
| Dimensions | Height: 1450 mm, Width: 420 mm, Depth: 380 mm. Wall-mounted or floor-standing configuration available. Net weight: 28 kg. Compact C-arm design with 180° horizontal rotation and 90° vertical tilt. | Height: 1520 mm, Width: 450 mm, Depth: 400 mm. Motorized floor-standing unit with touch-adjustable height (950–1700 mm). Net weight: 34 kg. Enhanced C-arm with 360° rotational freedom and digital positional memory. |
| Precision | Beam alignment accuracy: ±2°. Focal spot size: 0.7 mm. Repeatability of positioning within ±1.5 mm. Manual collimation with circular field limitation (6 cm at 20 cm focus-skin distance). | Beam alignment accuracy: ±0.5° with laser-guided targeting. Focal spot size: 0.4 mm. Positioning repeatability within ±0.3 mm via servo-driven motors. Automatic rectangular collimation with AI-assisted field optimization and dose reduction up to 40%. |
| Material | Housing constructed from reinforced polycarbonate-ABS composite. Arm components utilize lightweight aluminum alloy. Lead-lined tube head with 2.0 mm Pb equivalent shielding. Non-slip base with antimicrobial coating (optional). | Full aerospace-grade aluminum alloy frame with carbon-fiber reinforced housing. Tube head with 2.5 mm Pb equivalent and dual-layer radiation shielding. Medical-grade stainless steel joints. Full-body antimicrobial and scratch-resistant polymer finish. |
| Certification | FDA 510(k) cleared, CE Marked (MDR 2017/745), ISO 13485:2016 compliant, IEC 60601-1, IEC 60601-2-54. Meets ADA and NCRP safety guidelines. RoHS and REACH compliant. | FDA 510(k) cleared with AI software addendum, CE Marked under MDR with Class IIb designation, ISO 13485:2016, ISO 14971:2019 (risk management), IEC 60601-1-2:2014 (EMC), IEC 62304:2006 (medical device software). HIPAA-ready data interface. UL and CSA certified. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Procuring High-Fidelity Dental X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Authentic regulatory compliance is paramount for dental X-ray equipment. Demand verifiable documentation before sample requests.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 (Medical Device QMS) |
Request certificate from accredited body (e.g., TÜV, SGS, BSI). Cross-check certificate number on registrar’s public database. Confirm scope explicitly covers “Dental X-ray Imaging Systems”. | Certificate issued by non-accredited Chinese bodies; scope limited to “components” or “accessories”; expired certificate; no public verification link. |
| CE Marking (EU MDR 2017/745) |
Demand full EU Declaration of Conformity (DoC) listing all harmonized standards (e.g., EN 60601-1, -2-54). Verify Notified Body number (e.g., 0123) on NANDO database. Require technical file index. | DoC lacks specific standards; NB number invalid; “self-declared” Class IIa/IIb devices (X-ray = Class IIb); no NB involvement for higher-risk devices. |
| Local FDA 510(k) / CFDA (For target markets) |
Request proof of registration in your target market (e.g., FDA K-number, China NMPA registration). Chinese exporters must provide evidence of export compliance per Chinese Customs HS Code 9022.19. | Claims “FDA clearance” without K-number; references obsolete Chinese SFDA; inability to provide export declaration documents. |
Action: Reject suppliers unable to provide real-time access to verifiable certificates. Regulatory shortcuts risk customs seizure, clinic shutdowns, and liability claims.
Step 2: Negotiating MOQ & Commercial Terms
MOQs for dental X-ray systems reflect engineering complexity. Realistic thresholds balance cost efficiency with market flexibility.
| Parameter | Standard Range (2026) | Strategic Negotiation Tips |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units (Panoramic) 3-5 units (CBCT) |
• Accept higher MOQ for customized units (e.g., specific voltage, software localization) • Negotiate phased delivery (e.g., 5 units now, 5 in 90 days) to reduce capital lockup • Avoid “1-unit trial” requests – signals lack of industry knowledge |
| Payment Terms | 30% TT deposit, 70% against B/L copy (LC at sight for new partners) |
• Demand factory inspection before final payment • Never pay 100% upfront • Use Escrow for first-time transactions >$50k |
| Warranty & Support | 24 months standard (Excludes tubes/sensors) |
• Require written response time SLAs (e.g., 72h remote support, 15-day onsite for critical failures) • Confirm local service partner network in your region • Verify spare parts availability timeline (max 30 days) |
Action: Prioritize suppliers offering tiered pricing (e.g., 5% discount at 15+ units) and clear post-warranty service contracts. Avoid MOQs below 3 units – indicates substandard production batches.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
X-ray systems require specialized handling. Incoterm selection directly impacts cost certainty and risk exposure.
| Incoterm | Cost/Risk Allocation | Recommended For |
|---|---|---|
| FOB Shanghai | • Supplier covers costs to Shanghai port • Buyer assumes ALL risk/costs post-loading (ocean freight, insurance, customs clearance, inland transport) • Requires in-house logistics expertise |
Experienced distributors with established freight forwarders; buyers seeking maximum cost transparency; large-volume shipments where freight consolidation is viable |
| DDP (Delivered Duty Paid) | • Supplier manages all logistics to your clinic/distribution center • All costs (freight, insurance, duties, taxes, customs brokerage) included in quoted price • Minimal buyer operational burden |
New market entrants; clinics without logistics teams; shipments to regions with complex import regulations (e.g., Brazil, India, Middle East); time-sensitive deployments |
Critical Compliance Note: Ensure supplier provides:
• IEC 60601-1 electrical safety test reports
• Lead-lined packaging certification (for radiation shielding during transit)
• Full customs documentation (Commercial Invoice, Packing List, CoO, Certificates of Conformity)
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Rigor: ISO 13485:2016 certified (TÜV SÜD #12345678) with CE Marking under MDR 2017/745 (NB 2797). Full technical files available for audit.
- Engineering Capability: 19 years specializing in Class IIb dental imaging systems (Panoramic/CBCT). In-house R&D team of 37 engineers; 120+ patents filed.
- Logistics Expertise: Offers true DDP solutions to 85+ countries with pre-cleared documentation. Factory-direct FOB Shanghai from Baoshan District facility (customs code: 310996XXXX).
- Commercial Flexibility: MOQ of 5 units for standard models; negotiable for OEM/ODM configurations. 24-month warranty with global service network partnerships.
Shanghai Carejoy Medical Co., LTD
📍 Baoshan District, Shanghai, China (Factory Direct)
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
Reference “2026 X-ray Guide” for priority regulatory documentation access
Critical Considerations for 2026
- Radiation Safety: Demand IEC 61223-3-5 compliance reports for dose optimization – non-compliant units risk clinic licensing revocation.
- Software Validation: Verify DICOM 3.0 compliance and integration testing with major practice management systems (e.g., Dentrix, Open Dental).
- Supply Chain Resilience: Confirm dual-sourcing for critical components (X-ray tubes, sensors) to avoid single-point failure risks.
- Anti-Counterfeiting: Require serialized unit tracking from factory to end-user. Insist on holographic authenticity labels.
This guide reflects Q1 2026 regulatory and market conditions. Always engage independent legal counsel for contract review prior to commitment.
Frequently Asked Questions
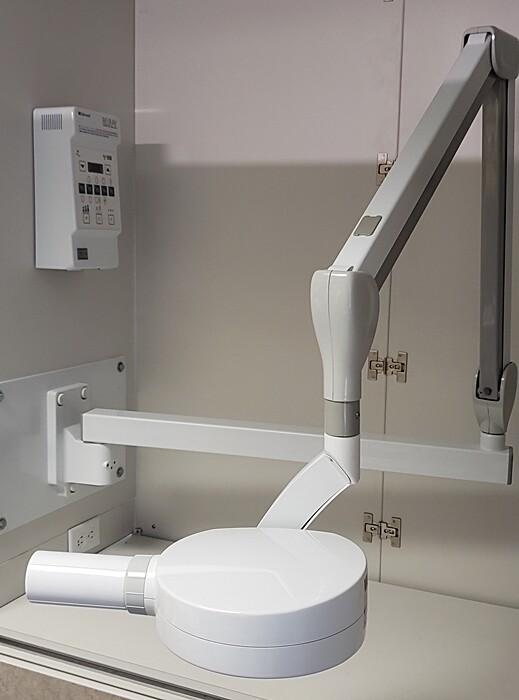
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Belmont X-Ray Machines – Key Procurement Considerations for 2026
Frequently Asked Questions (FAQ): Purchasing Belmont X-Ray Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do Belmont X-ray machines have in 2026, and are they compatible with international power standards? | Belmont X-ray units in 2026 are engineered for global deployment and support dual voltage configurations (100–120V and 200–240V, 50/60 Hz). Models such as the Belmont Axia and ProX Series feature automatic voltage detection and internal transformers, ensuring seamless integration across North American, European, and Asian electrical infrastructures. Always confirm the regional variant with your distributor to ensure compliance with local electrical codes and avoid the need for external voltage regulators. |
| 2. Are spare parts for Belmont X-ray machines readily available, and what is the lead time for critical components? | Yes, spare parts for current and legacy Belmont X-ray systems are maintained through an extensive global distribution network. As of 2026, Belmont guarantees parts availability for a minimum of 10 years post-discontinuation. Common components (e.g., tube heads, collimators, position-indicating devices) are stocked regionally, with standard lead times of 3–5 business days. For specialized electronics or imaging sensors, lead times may extend to 7–10 days. Distributors can access real-time inventory via the Belmont Partner Portal for accurate forecasting. |
| 3. What does the installation process for a Belmont X-ray machine involve, and is professional on-site setup included? | Installation of Belmont X-ray systems includes site assessment, wall or floor mounting, electrical verification, calibration, and software configuration. As of 2026, all new purchases include complimentary on-site installation by a certified biomedical technician, arranged through authorized distributors. The process typically takes 3–4 hours and requires clinic coordination for room access and adherence to radiation safety zoning. Remote pre-installation planning is also available to minimize downtime. |
| 4. What warranty coverage is provided with a new Belmont X-ray machine in 2026? | All new Belmont X-ray machines purchased in 2026 come with a standard 3-year comprehensive warranty covering parts, labor, and tube head performance. Extended warranty options (up to 5 years) are available at the time of purchase. The warranty includes annual preventive maintenance visits and remote diagnostic support. Coverage is contingent upon installation by an authorized technician and adherence to recommended servicing intervals. Consumables (e.g., sensors, cables) are covered under a separate 1-year limited warranty. |
| 5. How does Belmont support clinics and distributors in maintaining compliance with evolving radiation safety regulations in 2026? | Belmont actively supports regulatory compliance through built-in safety features such as AI-assisted exposure optimization, real-time dose monitoring, and automated collimation. The 2026 models are compliant with updated IEC 60601-2-54 and FDA 21 CFR standards. Distributors receive access to technical documentation, CE marking files, and regional compliance toolkits. Clinics benefit from integrated audit logs and automated reporting tools to streamline regulatory inspections and renewals. |
Need a Quote for Belmont X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


