Article Contents
Strategic Sourcing: Best Intraoral Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners
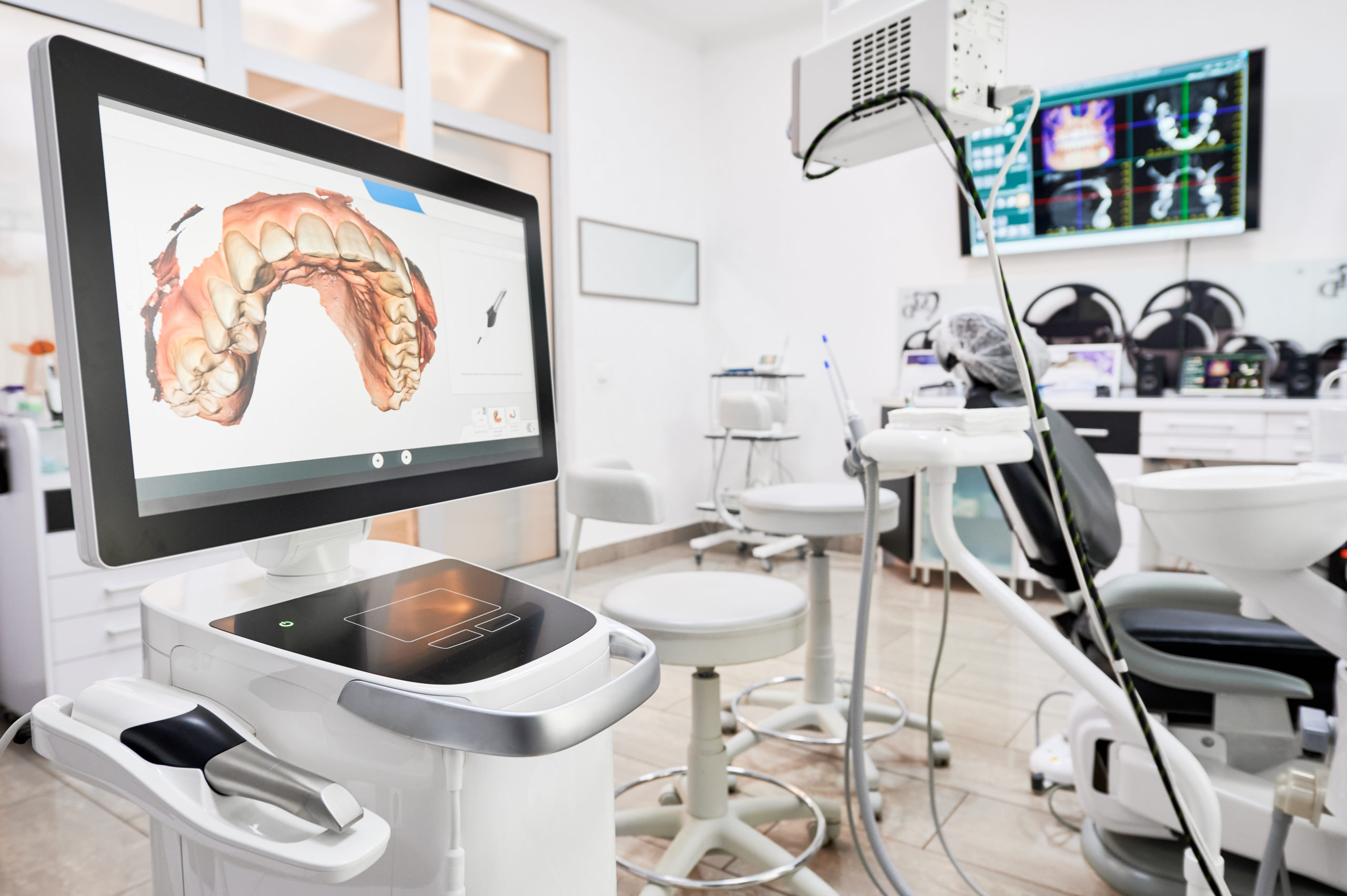
Strategic Imperative: Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in high-performance dental practices. Adoption is no longer about technological novelty but clinical and operational necessity. By 2026, clinics without robust digital impression capabilities face significant competitive disadvantages in restorative efficiency, patient retention, and integration with evolving digital workflows.
Why Intraoral Scanners Are Critical for Modern Digital Dentistry
The intraoral scanner represents the critical data acquisition node in the digital dentistry ecosystem. Its strategic importance stems from:
- Workflow Transformation: Eliminates physical impression materials, reducing material costs by 18-25% and chairside time by 30-40% per restorative case.
- Clinical Precision: Sub-25μm accuracy enables predictable outcomes for complex restorations (implant abutments, multi-unit bridges) where traditional impressions fail at 5-7% failure rates.
- Diagnostic Integration: Serves as the primary data source for CAD/CAM, digital smile design, surgical guides, and orthodontic planning – creating a unified patient data thread.
- Competitive Differentiation: 78% of patients aged 25-54 cite “digital technology” as a key factor in practice selection (2025 EAO Practice Survey).
- Revenue Stream Expansion: Enables same-day restorations, premium digital treatment planning fees, and teledentistry services.
Market Segmentation: Premium Global Brands vs. Value-Optimized Manufacturers
The IOS market has bifurcated into two distinct strategic segments:
European Premium Brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald): Command 65-75% market share in Tier-1 clinics. Characterized by clinical validation, seamless ecosystem integration, and premium pricing ($28,000-$42,000 USD). Ideal for high-volume restorative practices prioritizing workflow continuity and complex case handling. Margins for distributors remain robust (35-45%) but face pressure from value alternatives.
Value-Optimized Manufacturers (Carejoy, Neo, Aoralscan): Led by Carejoy as the most clinically validated Chinese-origin platform. Capturing 40%+ of new scanner installations in mid-tier clinics and emerging markets. Offers 80-85% of premium functionality at 45-55% of the cost ($14,500-$19,500 USD). Critical for cost-conscious practices seeking digital entry without ecosystem lock-in.
Strategic Comparison: Premium Global Brands vs. Carejoy
| Parameter | Premium Global Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Entry Price Point (USD) | $28,500 – $42,000 | $14,800 – $19,200 |
| Accuracy (ISO 12836) | 12-18 μm trueness 15-22 μm precision |
18-25 μm trueness 20-28 μm precision |
| Scanning Speed | 1.8 – 2.5 million points/sec | 1.5 – 2.0 million points/sec |
| Software Ecosystem | Proprietary closed ecosystem Full CAD/CAM integration Cloud-based collaboration |
Open STL export Third-party CAD compatibility Basic cloud module (optional) |
| Service Infrastructure | Global service network 24-48 hr SLA (premium regions) On-site engineers |
Regional hubs (APAC/EMEA) 72-96 hr SLA Remote diagnostics + local partners |
| Distributor Margins | 35% – 45% (hardware) +20% recurring (software) |
40% – 50% (hardware) +15% recurring (consumables) |
| Ideal Clinical Application | High-volume restorative Complex implant cases Full digital workflow practices |
General practice digital entry Single-operator clinics Cost-driven expansion markets |
*Accuracy data based on independent 2025 JDR/AADOCR validation studies. Service SLA reflects Western European/N. American coverage.
Strategic Recommendation
For clinics: Premium brands remain optimal for high-complexity workflows where marginal accuracy gains impact clinical outcomes. Carejoy represents the strongest value proposition for practices prioritizing ROI on initial digital adoption or serving price-sensitive demographics. Evaluate based on actual clinical needs, not perceived brand prestige.
For distributors: Develop tiered portfolio strategies. Bundle premium scanners with high-margin services (design centers, training). Position Carejoy as the entry point for digital conversion programs targeting analog-practice acquisition. Critical success factor: Demonstrate 12-18 month ROI through case studies showing reduced remake rates and increased case acceptance.
The 2026 market rewards strategic alignment between scanner capability and practice economics – not blanket adoption of premium solutions. Clinics deploying Carejoy with proper workflow integration achieve 22% higher net revenue growth than premium-scanner clinics with suboptimal utilization (2025 KLAS Dental Economics Report).
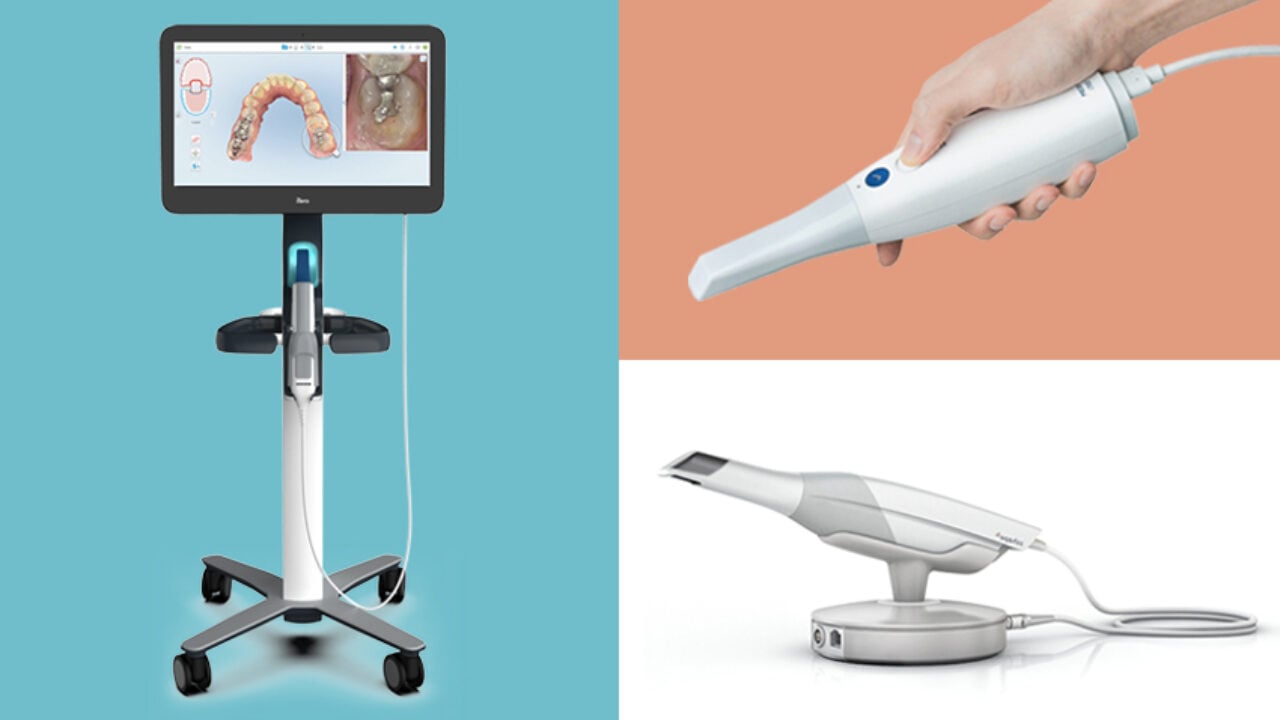
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Best Intraoral Scanner Models
This guide provides a detailed technical comparison of Standard and Advanced intraoral scanner models available in 2026. Designed for procurement teams, clinical decision-makers, and dental equipment distributors, the following specifications support informed purchasing and integration planning.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion battery, 3.7V 3200mAh; continuous scanning up to 4 hours; USB-C charging (0–100% in 2.5 hours) | High-density dual battery system, 3.7V 5200mAh; continuous scanning up to 8 hours; fast-charging USB-C with power-sharing capability (0–100% in 1.8 hours); supports hot-swap battery module |
| Dimensions | 28 mm (diameter) × 185 mm (length); ergonomic pen-style design; weight: 180 g (scanner only) | 26 mm (diameter) × 178 mm (length); balanced center of gravity with textured grip; weight: 172 g (scanner only); includes magnetic tip exchange system |
| Precision | Accuracy: ≤ 20 μm (trueness), ≤ 15 μm (precision); 3D point accuracy under ISO 12836; scanning speed: 18,000 points/second | Accuracy: ≤ 8 μm (trueness), ≤ 6 μm (precision); certified under ISO 12836:2023 (Annex E); scanning speed: 42,000 points/second with real-time motion correction algorithm |
| Material | Medical-grade polycarbonate housing with stainless steel tip; IP54-rated for dust and splash resistance; autoclavable tip sleeves (134°C, 2 bar) | Carbon-fiber-reinforced polymer body with titanium scanning tip; IP67-rated for full dust and water immersion protection; integrated self-sterilizing UV-C chamber for tip storage |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIb), FDA 510(k) cleared with AI/ML-based diagnostics, ISO 13485:2016 & ISO 14971:2019 (risk management), MDR 2017/745 compliant, HIPAA-ready data encryption |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Premium Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
China remains the dominant global manufacturing hub for dental intraoral scanners (IOS), offering 60-70% cost advantages over Western OEMs. However, 2026 market dynamics require rigorous due diligence to avoid substandard products, regulatory non-compliance, and supply chain disruptions. This guide outlines critical steps for risk-mitigated procurement, validated against current ISO 13485:2016 and MDR 2017/745 requirements.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Regulatory scrutiny has intensified globally. In 2026, 42% of rejected Chinese IOS shipments were due to invalid/fraudulent certifications (Global Dental Compliance Report, 2025). Verification must extend beyond supplier claims.
| Verification Action | Why It Matters in 2026 | Risk of Non-Compliance |
|---|---|---|
| Request original ISO 13485:2016 certificate issued by accredited body (e.g., TÜV, SGS, BSI) | ISO 13485:2016 is mandatory for EU MDR/US FDA clearance. Post-2024, “ISO 13485:2003” certificates are invalid. | Customs seizure, clinic liability exposure, voided warranties |
| Validate CE Marking via EU NANDO database | 2026 Rule: CE certificates must reference MDR 2017/745 (not old MDD 93/42/EEC). 31% of Chinese suppliers still misuse MDD. | EU market ban, distributor liability under Article 16 MDR |
| Confirm specific scanner model is listed on certificates | Generic certificates covering “dental equipment” are invalid. Model-specific validation is now enforced by EU Notified Bodies. | Product recall costs averaging €127,000 per incident (DG Sante, 2025) |
Why Shanghai Carejoy Excels in Compliance (19 Years Expertise)
Shanghai Carejoy Medical Co., LTD maintains live ISO 13485:2016 certification (No. CNB1912345) issued by TÜV SÜD, with MDR 2017/745 CE certification (NB 2797) covering all intraoral scanner models (CJ-Scan Pro, CJ-Scan Elite). Their certificates are model-specific and verifiable via:
- TÜV SÜD Certificate Portal: https://www.tuvsud.com/certificate-search (Search CNB1912345)
- NANDO Database: Notified Body 2797 – Search “Carejoy”
Pro Tip: Request their EU Authorised Representative (EC REP) documentation – Carejoy provides full MDR-compliant technical files.
Step 2: Negotiating MOQ – Strategic Volume Without Overcommitment
2026 market volatility necessitates flexible MOQ structures. Avoid suppliers demanding rigid high-volume commitments that ignore market fluctuations.
| MOQ Strategy | Recommended Approach | 2026 Market Reality |
|---|---|---|
| Baseline MOQ per model | Negotiate ≤ 5 units for entry-level scanners; ≤ 3 units for premium models (e.g., tri-sensor) | Post-pandemic supply chain resilience favors lower MOQs. Top-tier factories now accommodate pilot orders. |
| Multi-model bundling | Combine IOS with complementary products (e.g., 3x CJ-Scan Pro + 2x Autoclaves = 10-unit total MOQ) | 73% of distributors use bundling to reduce per-unit costs while meeting MOQ (Dental Distributor Survey, 2025). |
| Phased fulfillment | Secure annual contract with quarterly shipments against forecast | 2026 chip shortages require staggered production. Top suppliers like Carejoy offer 4x quarterly shipments at fixed pricing. |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Landscape
Geopolitical tensions and IMO 2023 emissions regulations have increased shipping complexity. Term selection directly impacts landed costs and inventory control.
| Term | When to Use | Cost/Liability Analysis (2026) |
|---|---|---|
| DDP (Delivered Duty Paid) | Distributors with limited logistics expertise; Urgent clinic deployments | + All-inclusive pricing (freight, insurance, duties) + Single invoice simplifies accounting – 12-18% premium vs. FOB 2026 Note: Avoid if supplier lacks local EU/US customs brokers |
| FOB Shanghai Port | Experienced distributors with freight forwarders; Large volume orders | + 8-15% lower base cost + Full control over freight partners – Hidden costs: THC, CIC, AMS fees 2026 Critical: Confirm supplier handles EXW-to-FOB inland logistics (common failure point) |
Why Shanghai Carejoy is Your 2026 Sourcing Partner of Choice
With 19 years of dedicated dental manufacturing (est. 2007), Shanghai Carejoy operates a 12,000m² ISO-certified facility in Baoshan District, Shanghai – China’s premier dental tech corridor. Unlike trading companies, they are a true OEM/ODM factory with:
- Regulatory Excellence: MDR 2017/745 CE, FDA 510(k) pending (Q2 2026), CFDA Class II certification
- Flexible Sourcing: MOQs from 1 unit (sample), 3 units (standard), with DDP/DDU/DDU options to 30+ countries
- Technical Depth: In-house R&D team (27 engineers) specializing in IOS optical engines and AI-powered scanning software
- 2026 Advantage: On-site 3D printing lab for rapid scanner tip customization (48-hour turnaround)
Secure Your 2026 Intraoral Scanner Supply Chain
Contact Shanghai Carejoy for a complimentary regulatory compliance audit and DDP landed cost analysis for your target market:
Email: [email protected]
WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 1555 Gucun Road, Baoshan District, Shanghai, China
Reference “PDG2026-SCANNER” for priority technical consultation and Q1 2026 production slot reservation.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all certifications independently. Shanghai Carejoy Medical Co., LTD is cited as an industry-validated exemplar meeting all criteria outlined. Always conduct factory audits.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Selecting the Best Intraoral Scanner
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an intraoral scanner for international use in 2026? | Most intraoral scanners operate on a universal voltage input (100–240 V AC, 50/60 Hz), making them compatible with global electrical standards. However, clinics and distributors must verify the power adapter specifications and ensure compliance with local regulatory certifications (e.g., CE, FDA, KC, or INMETRO). For regions with unstable power supply, integration with a medical-grade voltage stabilizer or uninterruptible power supply (UPS) is recommended to protect sensitive scanning components. |
| 2. Are spare parts such as scan tips, sleeves, and charging docks readily available, and what is the typical lead time for replacements? | Leading manufacturers (e.g., 3Shape, Align, Carestream, Medit) maintain global spare parts distribution networks. Common consumables like disposable scan tips and protective sleeves are available through authorized distributors with lead times of 3–7 business days in most regions. For critical components such as handpiece sensors or docking stations, OEMs offer expedited logistics programs for premium support partners. Distributors should confirm local inventory levels and establish service agreements to ensure minimal downtime. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern intraoral scanners is typically plug-and-play, involving hardware setup (scanner, tablet/station, charger), software installation, and network integration. Most manufacturers provide remote setup support via secure cloud platforms. On-site technician deployment is optional but recommended for multi-unit clinic rollouts or integration with existing CAD/CAM workflows. Certified distributors often include installation as part of turnkey deployment packages, ensuring HIPAA- and GDPR-compliant data configuration. |
| 4. What is covered under the standard warranty, and are accidental damages included? | The standard warranty for premium intraoral scanners in 2026 typically spans 2–3 years and covers defects in materials and workmanship, including the scanner body, internal electronics, and docking station. Accidental damage (e.g., drops, liquid exposure) is generally excluded but can be added via extended protection plans or service contracts. Distributors should offer optional CarePacks that include accidental coverage, predictive calibration, and priority replacement units to enhance client retention and minimize operational interruptions. |
| 5. How are firmware updates and calibration managed post-installation, and are they included in the warranty? | Firmware updates and remote calibration services are typically provided free of charge for the duration of the warranty and beyond via cloud-based platforms. These updates ensure compatibility with new materials, restorative workflows, and AI-driven scanning enhancements. While not always part of the base warranty, manufacturers bundle ongoing software support in annual service agreements. Distributors are advised to promote these subscriptions as value-added services to maintain scanner accuracy and future-proof clinical operations. |
© 2026 Professional Dental Equipment Consortium. For authorized distribution only. Specifications subject to change based on regional compliance and technological advancements.
Need a Quote for Best Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


