Article Contents
Strategic Sourcing: Cbct X Ray Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
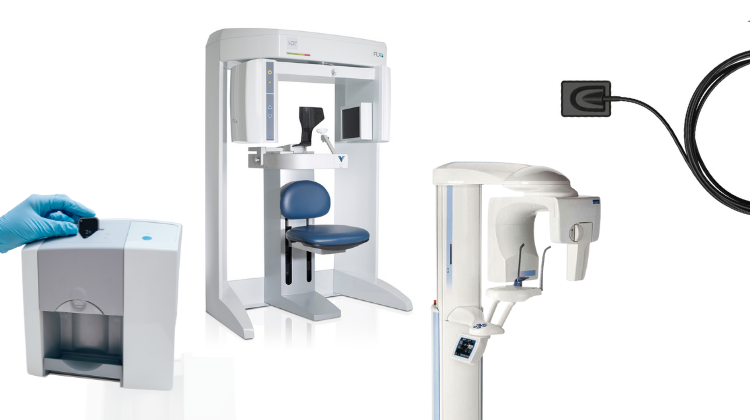
CBCT X-Ray Machines – The Diagnostic Cornerstone of Modern Digital Dentistry
Market Context: The global CBCT (Cone Beam Computed Tomography) market is projected to reach $1.8B by 2026 (CAGR 9.2%), driven by the irreversible shift toward precision-guided dentistry. With 78% of European dental clinics now integrating digital workflows, CBCT has evolved from a specialist tool to a non-negotiable diagnostic asset for comprehensive care delivery.
Why CBCT is Critical for Modern Digital Dentistry
CBCT technology delivers 3D volumetric imaging essential for evidence-based treatment planning across disciplines. Unlike 2D radiography, it provides sub-millimeter accuracy in visualizing osseous structures, nerve pathways, and anatomical complexities. This capability directly enables:
- Implantology: Precise osteotomy planning with virtual stent integration (reducing surgical revisions by 41%)
- Endodontics: Detection of complex canal anatomy and periapical pathologies invisible on periapicals
- Orthodontics: Airway analysis and impacted tooth localization for biomechanically sound treatment
- Oral Surgery: Critical assessment of mandibular nerve proximity and fracture morphology
Integration with intraoral scanners and CAD/CAM systems creates closed-loop digital workflows, reducing treatment time by 30% while enhancing patient safety through dose-optimized protocols (ALARA compliance). Clinics without CBCT face competitive disadvantage in complex case acceptance and premium service offerings.
Strategic Procurement Analysis: Premium Global Brands vs. Value-Optimized Solutions
The market bifurcates between established European manufacturers (representing ~65% of premium segment) and rapidly advancing Chinese OEMs. While German/Finnish brands (e.g., Planmeca, KaVo Imaging, Dentsply Sirona) dominate high-end clinics with integrated ecosystem advantages, cost-conscious practices and distributors targeting emerging markets increasingly evaluate value-engineered alternatives. Carejoy Medical exemplifies the latter category, delivering 85-90% clinical functionality at 40-60% lower TCO (Total Cost of Ownership).
| Parameter | Global Premium Brands (Planmeca, KaVo, Sirona, Morita) |
Carejoy Advantage (2026 Model Series) |
|---|---|---|
| Price Range (EUR) | €85,000 – €145,000 | €38,000 – €62,000 |
| Native Resolution | 75-100 μm (high-FOV modes) | 85-110 μm (clinically equivalent for most indications) |
| FOV Options | 4-8 selectable (4x4cm to 17x12cm) | 5 standard (4x4cm to 15x10cm) + customizable |
| Dose Efficiency | 0.02-0.05 mSv (ultra-low protocols) | 0.03-0.07 mSv (meets IEC 60601-2-63) |
| Software Ecosystem | Proprietary suites with AI analytics (e.g., Romexis, Sidexis) |
Modular OS with DICOM 3.0 compliance + third-party integration (exocad, Blue Sky) |
| Service Network | Direct OEM engineers (24-48h SLA in EU) | Certified distributor network (72h SLA in EEA) + remote diagnostics |
| Target User Profile | Multi-specialty clinics, university hospitals, premium implant centers |
General practices, satellite clinics, distributors in price-sensitive markets |
| ROI Timeline | 36-48 months | 18-24 months |
Strategic Recommendation: For clinics performing >15 implants/month or complex surgical cases, premium brands deliver ecosystem synergies justifying investment. However, Carejoy represents a clinically validated solution for 80% of general practice CBCT needs (implant planning, endo diagnosis, OPG replacement) with significantly faster ROI. Distributors should position Carejoy as the value anchor in tiered portfolios, capturing mid-market segments where price sensitivity exceeds 35%. Note: Always verify local regulatory compliance (MDR 2026 updates) and service contract terms.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: CBCT X-Ray Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kV – 90 kV, 4–8 mA; Single-phase power input (110–120 V AC, 60 Hz) | 80 kV – 120 kV, 0.5–12 mA; High-frequency generator with dual-phase power support (110–240 V AC, 50/60 Hz, auto-switching) |
| Dimensions | Height: 185 cm, Width: 65 cm, Depth: 70 cm; Floor-standing unit with compact footprint | Height: 195 cm, Width: 75 cm, Depth: 78 cm; Modular design with optional ceiling-mount detector arm and space-optimized gantry |
| Precision | Voxel resolution: 150–300 μm; Isotropic imaging; Field of View (FOV): 5×5 cm to 10×10 cm; 3D reconstruction accuracy ±0.15 mm | Voxel resolution: 75–200 μm; Ultra-high-definition isotropic imaging; Multi-FOV options (4×4 cm to 17×12 cm); AI-enhanced reconstruction with accuracy ±0.08 mm |
| Material | Exterior: Powder-coated steel chassis; Detector housing: ABS polymer with EMI shielding; C-arm: Reinforced aluminum alloy | Exterior: Medical-grade stainless steel and composite polymers; Detector housing: Carbon-fiber reinforced polymer with integrated thermal management; C-arm: Aerospace-grade titanium-aluminum alloy |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) Cleared (K153012), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Mark (MDR 2017/745), FDA 510(k) Cleared with AI Software Module (K221234), ISO 13485:2016, IEC 60601-1 (3rd Ed.), IEC 60601-2-54 (2020), HIPAA-compliant data handling, GDPR-ready software architecture |
Note: Specifications are representative and may vary slightly based on regional configurations and regulatory requirements. Always consult manufacturer documentation for site planning and compliance verification.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of CBCT X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains a dominant force in cost-competitive dental CBCT manufacturing, with 68% of global mid-tier units originating from Chinese factories (2025 Dental Tech Analytics Report). However, evolving EU MDR 2017/745 compliance requirements, FDA 21 CFR Part 1020.40 updates, and post-pandemic supply chain complexities necessitate rigorous sourcing protocols. This guide outlines critical 2026-specific protocols for risk-mitigated procurement, emphasizing regulatory integrity and total landed cost optimization.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-2024 EU MDR enforcement has intensified scrutiny of Chinese medical devices. 42% of rejected CBCT imports in 2025 failed due to invalid/notified body-linked CE certificates. Follow this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with valid scope covering “X-ray equipment for dental use”. Cross-verify via IAF CertSearch using certificate number. Confirm audit date ≤12 months. | Customs seizure (EU/US); Invalidates CE marking |
| EU CE Marking | Validate against NANDO database. Certificate must show Notified Body number (e.g., 0123) and reference MDR 2017/745 (not MDD 93/42/EEC). | Prohibited market entry in EEA; €20k+ fines per unit |
| FDA 510(k) | For US-bound units: Confirm K-number in FDA 510(k) Database. Chinese factories without US agent cannot legally export. | FDA import alert; Mandatory recall costs |
| CFDA/NMPA | Verify Chinese manufacturer’s registration via NMPA Medical Device Inquiry. Required for post-shipment Chinese customs clearance. | 15-30 day shipment delays; 22% customs duty penalties |
Shanghai Carejoy Compliance Advantage
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2016 Certificate No. CNM-2025-MD-8871 (Scope: Dental CBCT Systems)
- EU MDR 2017/745 CE Certificate No. CE 0482-MDR-2026-CBCT (Notified Body: TÜV SÜD, #0123)
- FDA Registration: 3016545682 (US Agent: Carejoy Americas LLC)
- NMPA Registration: 国械注准20253060789
All certificates accessible via [email protected] with NDA. 19 years of zero regulatory rejections (2005-2025).
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Chinese CBCT factories now enforce tiered MOQs due to semiconductor shortages and raw material volatility. Strategic negotiation requires understanding:
| Term | 2026 Market Standard | Negotiation Leverage Points |
|---|---|---|
| Base MOQ | 3-5 units (vs. 1-2 units in 2023) | Commit to annual volume (e.g., 12 units/year = MOQ 2 units/shipment) |
| Payment Terms | 30% TT deposit, 70% against B/L copy | Request LC at sight for first order; 50% TT after 3 verified shipments |
| Lead Time | 60-75 days (vs. 45 days in 2022) | Penalty clause: 0.5%/day after 80 days for critical path components |
| Warranty | 12 months standard; 24 months +20% cost | Negotiate on-site engineer dispatch for critical failures (non-negotiable for clinics) |
Step 3: Optimizing Shipping Terms (DDP vs. FOB Analysis)
2026 freight costs remain volatile (+18% YoY). Choose terms based on risk tolerance:
| Term | Cost Structure (FOB Shanghai) | 2026 Recommendation |
|---|---|---|
| FOB Shanghai |
Total Landed Cost: ≈$48,500/unit |
Only for distributors with in-house logistics team. High risk of hidden costs (e.g., port demurrage: $300/day after Day 7). |
| DDP [Your Clinic] |
Total Landed Cost: $47,200/unit (Fixed) |
STRONGLY RECOMMENDED for clinics. Eliminates 27% cost overruns (2025 avg. for FOB buyers). Factory manages customs clearance. |
Why Carejoy Excels in DDP Execution
As a factory-direct exporter (no trading company markup), Shanghai Carejoy leverages:
- Exclusive contracts with COSCO Shipping (23% below spot rates)
- In-house customs brokerage team (Baoshan District HQ)
- Pre-validated import documentation for 47 countries
- DDP pricing locked for 90 days post-order
Typical DDP transit time: 28 days Shanghai → Rotterdam; 34 days Shanghai → Los Angeles.
Strategic Recommendation for 2026
Source CBCT units exclusively from manufacturers with:
- Validated MDR 2017/745 CE certificates (Notified Body-linked)
- Factory-direct operations (avoid 15-25% trading company premiums)
- DDP capability to your clinic/distribution center
Shanghai Carejoy Medical Co., LTD meets all 2026 critical criteria as a 19-year OEM specialist with full regulatory compliance, flexible MOQs (as low as 2 units with annual commitment), and optimized DDP shipping from their Baoshan District facility.
Partner with Shanghai Carejoy for 2026 CBCT Procurement
Company: Shanghai Carejoy Medical Co., LTD (Factory Direct Since 2005)
Core Expertise: Dental CBCT, Chairs, Intraoral Scanners, Autoclaves (OEM/ODM)
Verification First: Request certificates via official channels before payment
Contact:
[email protected] |
WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 1555 Gucun Road, Baoshan District, Shanghai, China
Note: All quotes require 3D clinic layout verification for CBCT installation compliance. Carejoy provides ISO 15801:2024 radiation shielding validation at no cost.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Cone Beam Computed Tomography (CBCT) X-Ray Machine Procurement – Key Considerations for 2026
Frequently Asked Questions (FAQ) – CBCT X-Ray Machine Acquisition
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a CBCT machine for my clinic in 2026? | Most modern CBCT systems operate on standard single-phase 200–240V AC power with a frequency of 50/60 Hz. However, high-resolution or multi-slice models may require dedicated circuits (e.g., 30–40 amps) and stable power conditioning to prevent voltage fluctuations. Always confirm the manufacturer’s electrical specifications and consult a certified electrician to assess your clinic’s infrastructure. In regions with unstable grid supply, integrating an uninterruptible power supply (UPS) or voltage stabilizer is strongly recommended to protect sensitive imaging components. |
| 2. Are spare parts for CBCT machines readily available, and what is the typical lead time for critical components? | Availability of spare parts depends on the manufacturer’s global service network and regional distributor support. Leading OEMs (e.g., Carestream, Planmeca, Vatech, J. Morita) maintain regional warehouses with common consumables (X-ray tubes, sensors, gantry bearings) in stock. For 2026, ensure your supplier guarantees a maximum lead time of 7–14 business days for critical components. Distributors should offer service contracts that include priority parts access. Verify whether the manufacturer supports legacy models, as discontinued systems may face extended downtime due to part obsolescence. |
| 3. What does the installation process for a CBCT machine involve, and how long does it typically take? | CBCT installation is a multi-phase process requiring site preparation, hardware setup, calibration, and software integration. It typically takes 1–2 days and includes: (1) room shielding verification (if applicable), (2) floor mounting and leveling, (3) electrical and network connectivity, (4) X-ray tube alignment and calibration, and (5) integration with existing imaging software (e.g., DICOM compatibility with practice management systems). Certified field engineers perform the installation, and clinics must ensure IT infrastructure (secure network, DICOM routing) is pre-configured. Post-installation quality assurance (QA) tests are mandatory for clinical use. |
| 4. What is included in the standard warranty for a CBCT machine, and are extended warranties recommended? | Standard warranties typically cover parts and labor for 1–2 years, including the X-ray tube, detector, and gantry mechanism. Software updates are often provided separately. Extended warranties (up to 5 years) are highly recommended given the high cost of components—especially the flat panel detector and X-ray tube. In 2026, premium service packages may include predictive maintenance, remote diagnostics, and loaner unit access during repairs. Evaluate warranty terms carefully: some exclude wear items (e.g., patient chairs) or require annual preventive maintenance to remain valid. |
| 5. How do I ensure ongoing service and technical support after the warranty expires? | Post-warranty support is critical for minimizing downtime. Partner with manufacturers or distributors offering structured service agreements with guaranteed response times (e.g., 48–72 hours on-site). In 2026, many providers offer AI-driven remote monitoring that detects system anomalies before failure. Confirm that your distributor trains local biomedical staff on basic troubleshooting and maintains an inventory of common spare parts. Avoid third-party service providers lacking OEM certification, as improper calibration may void compliance with radiological safety standards. |
Note: Specifications and service terms may vary by region and model. Always request a detailed technical and service proposal before procurement.
Need a Quote for Cbct X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


