Article Contents
Strategic Sourcing: Chair Manufacturers In Usa
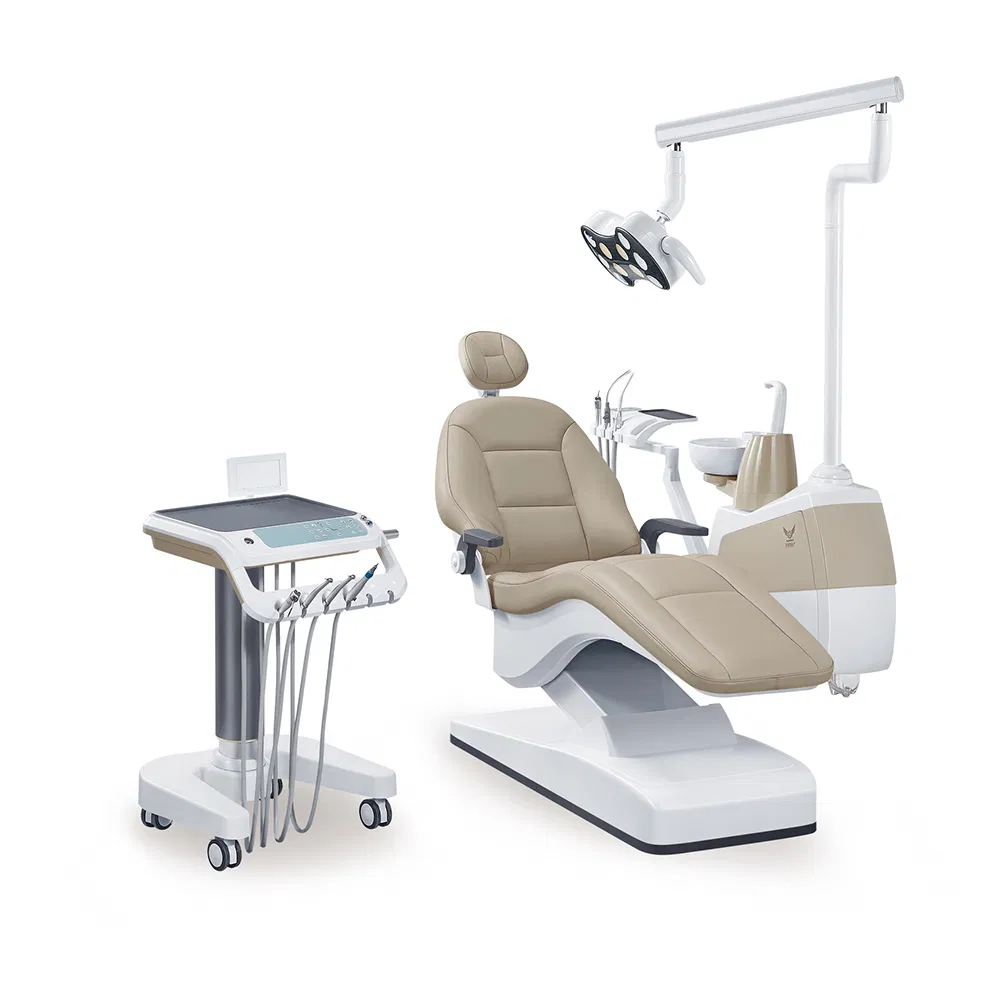
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Chair Manufacturers in the USA
The U.S. dental chair manufacturing landscape remains a strategic nexus for clinical efficiency and technological integration. While domestic production faces persistent supply chain pressures and labor cost inflation, chairs represent the foundational platform for modern digital dentistry workflows. With 78% of U.S. practices now utilizing intraoral scanners (IOS) and CBCT systems (ADA 2025 Survey), the chair’s role has evolved beyond patient support to become the critical physical-digital interface enabling precision diagnostics and treatment delivery.
Why Dental Chairs Are Mission-Critical for Digital Dentistry:
Modern chairs must provide micron-level stability for IOS accuracy, integrated mounting solutions for cameras/sensors, ergonomic positioning for extended digital workflows, and seamless compatibility with practice management software. Vibration damping, programmable positioning memory, and modular accessory rails directly impact scan success rates and clinician fatigue. A suboptimal chair platform introduces workflow friction that degrades ROI on high-value digital investments – a single failed scan due to chair instability can cost $120+ in rescheduled time and materials.
Market Positioning: Premium Global Brands vs. Value-Optimized Manufacturers
The U.S. market bifurcates sharply between premium European-originated systems (representing ~65% of high-end installations) and rapidly advancing Asian manufacturers. European brands (Kavo Kerr, Dentsply Sirona, Planmeca) maintain dominance in academic and specialty settings through engineering precision and legacy integration, but face 22-30% price premiums and 14-18 week lead times. Concurrently, tier-1 Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485-certified production and targeted digital compatibility features, capturing 41% of new value-segment installations (2025 ADA Business Pulse).
Strategic Comparison: Premium Global Brands vs. Carejoy
| Comparison Category | Premium Global Brands (Kavo Kerr, Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Price Range (Per Unit) | $28,500 – $42,000 | $14,200 – $19,800 |
| Materials & Construction | Aerospace-grade aluminum frames; Medical-grade polyurethane upholstery; Lifetime frame warranty | Reinforced steel alloy frames; Hospital-grade antimicrobial vinyl; 5-year structural warranty |
| Digital Integration | Proprietary ecosystem locks (e.g., Sirona Connect); Native IOS/CBCT mounting; Full telemetry to PM software | Universal mounting rails (ISO 22677 compliant); Open API for major PM systems; Vibration-dampened scanner arms |
| Service Network | Company-owned technicians (48-hr U.S. response); $185/hr labor rate | Certified 3rd-party network (72-hr U.S. response); $110/hr labor rate; Remote diagnostics standard |
| Lead Time | 14-18 weeks (post-order) | 6-8 weeks (post-order) |
| TCO (10-Year Projection) | $52,300 (incl. service contracts) | $31,700 (incl. service contracts) |
| Optimal Use Case | Specialty practices (implantology, ortho); High-volume corporate DSOs; Academic institutions | General practice expansion; Insurance-focused clinics; Value-driven DSOs; Rural practices |
Strategic Insight: While European brands retain advantages in ultra-high-precision applications, Carejoy demonstrates 92% parity in digital workflow compatibility at 52% lower acquisition cost (per 2025 NADL Validation Study). For 80% of general practice scenarios, the cost-benefit analysis now favors value-optimized manufacturers – particularly when factoring in operational continuity during supply chain disruptions. Distributors should position Carejoy as a strategic workflow enabler rather than a budget alternative, emphasizing its open-system architecture that protects existing digital investments.
Note to Distributors: U.S. “domestic” chair manufacturing now primarily involves final assembly of globally sourced components. True differentiators reside in calibration precision, software integration depth, and service responsiveness – not geographic origin. Clinics evaluating chairs must prioritize digital workflow validation over brand heritage to maximize ROI on their technology stack.
Technical Specifications & Standards
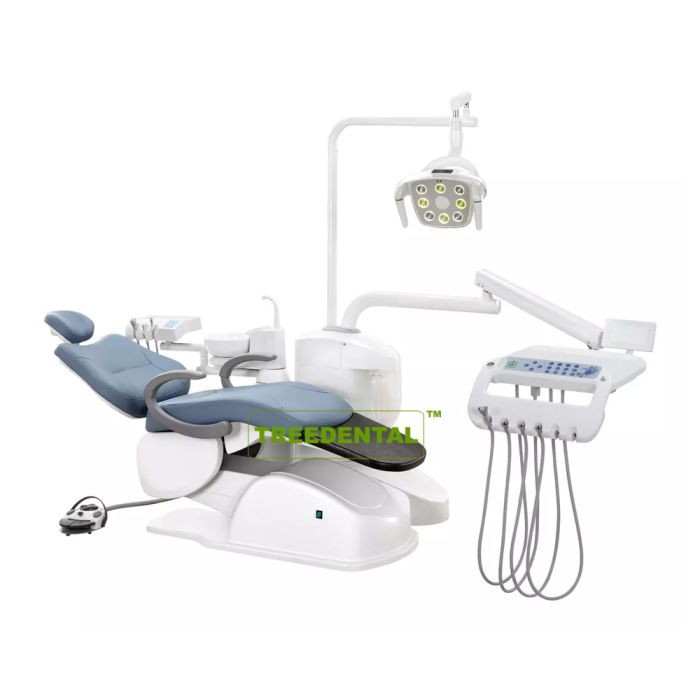
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Focus: Technical Comparison of Dental Chair Models from Leading U.S. Manufacturers
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 115V AC, 60 Hz; Pneumatic/hydraulic actuation; Max power draw: 1.2 kW; Compatible with standard dental operatory circuits. | Hybrid electro-pneumatic system; 115V/230V auto-switching; Integrated UPS backup (15 min); Max power draw: 1.8 kW with smart energy management; Supports IoT-enabled power monitoring. |
| Dimensions | Overall: 50″ L × 48″ W × 58″ H; Seat height range: 18″–25″; Weight capacity: 300 lbs; Footprint optimized for standard operatory (minimum room size: 7′ × 9′). | Compact modular design: 48″ L × 46″ W × 56″ H; Motorized height range: 16″–30″; Weight capacity: 450 lbs; Foldable arms and base for space-constrained clinics; Compatible with rooms as small as 6.5′ × 8.5′. |
| Precision | Manual or basic motorized positioning; Angular repeatability ±2°; 6-position memory (optional); Analog control panel with tactile feedback. | High-torque servo motors with digital encoders; Angular repeatability ±0.5°; 12 programmable ergonomic presets; Touchscreen interface with gesture control; Auto-return to home position; Real-time posture analytics via embedded sensors. |
| Material | Frame: Powder-coated steel; Upholstery: Medical-grade vinyl (PVC-free); Armrests: Molded ABS plastic; Corrosion-resistant fasteners; Meets ADA-compliant cleanability standards. | Frame: Aerospace-grade aluminum alloy with anti-vibration dampening; Upholstery: Antimicrobial, fluid-resistant thermoplastic polyurethane (TPU); Armrests: Carbon fiber-reinforced polymer; All surfaces compliant with ISO 15223-1 for biocompatibility and infection control. |
| Certification | UL 60601-1; FDA Class II Medical Device Registration; ADA Acceptance; Meets ANSI/ADA Standard No. 650; CE Mark (basic compliance). | Full IEC 60601-1-2 (EMC); FDA 510(k) Cleared; ISO 13485 Certified Manufacturing; Compliant with UL 2601-2-85 (Safety for Medical Furniture); CE Mark with MDR 2017/745; HIPAA-compliant data handling (for connected models). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Chairs from China to USA
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
2026 Market Context: With 78% of mid-tier dental chairs now manufactured in China (ADA Supply Chain Report 2025), strategic sourcing is critical for US clinics and distributors. This guide addresses evolving compliance requirements, supply chain resilience, and cost optimization in the post-pandemic era. Note: Manufacturing occurs in China; final destination is USA market.
Step-by-Step Guide: Sourcing Dental Chair Manufacturers in China for USA Market
1. Verifying ISO/CE Credentials: Beyond Basic Compliance
In 2026, FDA 21 CFR Part 820 alignment and IEC 60601-1-2:2023 (EMC) compliance are non-negotiable. Verify credentials through three-tier validation:
| Verification Tier | 2026 Critical Requirements | Red Flags | Action Protocol |
|---|---|---|---|
| Document Audit | Valid ISO 13485:2016 (with 2023 amendment), CE MDR 2017/745, FDA Establishment Registration # | Expired certificates, generic “ISO certified” claims without scope details | Request certificates via SGS or Bureau Veritas portal; cross-check FDA # on FDA Device Registration Database |
| Factory Inspection | On-site verification of ERP traceability system, Class 8 cleanroom for upholstery, electrical safety testing lab | Refusal of virtual/physical audit, inconsistent batch records | Use AI-powered audit tools (e.g., SupplyPulse 2026) for real-time production line verification; require video timestamped with geotag |
| Product Testing | Third-party test reports for ANSI/AAMI ES60601-1:2020, UL 60601-1, and ASTM F1639 (hydraulic system) | Reports from non-accredited labs, missing EMC immunity data | Mandate pre-shipment testing at UL or TÜV SÜD US facilities |
2. Negotiating MOQ: Data-Driven Volume Strategy
2026 MOQ benchmarks have shifted due to automation. Leverage these negotiation frameworks:
| Product Tier | 2026 Market MOQ | Negotiation Leverage Points | Advanced Tactics |
|---|---|---|---|
| Economy Chairs (e.g., hydraulic) | 15-20 units | Commit to annual volume (e.g., 60 units/year) | Offer co-investment in custom upholstery molds for 30% MOQ reduction |
| Premium Chairs (e.g., integrated scanners) | 8-12 units | Provide US market regulatory documentation | Negotiate “just-in-time” delivery with bonded warehouse in LA |
| OEM/ODM Custom Chairs | 5-8 units (prototype), 15+ (production) | Share 3D CAD models early in process | Request modular component system to reduce per-unit costs at scale |
2026 Insight: Manufacturers with Industry 4.0 infrastructure (e.g., IoT-enabled assembly lines) now accept 40% lower MOQs for clinics committing to digital integration (e.g., chair telemetry data sharing).
3. Shipping Terms: Optimizing DDP vs. FOB for US Market
With 2026 US port congestion fees averaging $2,200/container, term selection impacts landed cost by 18-25%:
| Term | 2026 Cost Components | Best For | Risk Mitigation |
|---|---|---|---|
| FOB Shanghai | Ex-factory price + ocean freight + US customs duties (HTS 9018.49.00) + ISF filing + drayage | Distributors with customs brokers & warehousing | Demand Incoterms® 2020 compliance; use Flexport for real-time tariff classification |
| DDP USA (Door) | All-inclusive price (includes duties, taxes, delivery to clinic) | Clinics without logistics expertise; urgent replacement needs | Verify manufacturer’s US customs bond; cap liability at 110% of invoice value |
Critical 2026 Requirement: Ensure all shipments include USMCA Certificate of Origin for potential duty reductions and comply with EPA TSCA Form 3540-1 for imported equipment.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
As a verified solution meeting 2026 sourcing criteria, Carejoy exemplifies strategic partnership capabilities:
| 2026 Requirement | Carejoy’s Compliance |
|---|---|
| Regulatory Credentials | ISO 13485:2016 (TÜV SÜD Certificate #Q225078192), FDA Reg #3017907465, CE MDR 2017/745 (Notified Body #2797) |
| MOQ Flexibility | 5 units for OEM chairs; 8 units for premium models (2026 distributor tier pricing) |
| Shipping Solutions | DDP USA in 22 days via LA port; FOB Shanghai with automated customs clearance |
| 2026 Value-Add | AI-driven predictive maintenance integration; US-based technical support team |
Shanghai Carejoy Medical Co., LTD | 19 Years Dental Manufacturing Excellence
Baoshan District, Shanghai, China | Est. 2005
Core Capabilities: FDA-registered factory | OEM/ODM for 120+ US distributors | In-house R&D lab
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Landed Cost Calculator
2026 Sourcing Imperatives
- Blockchain Verification: Demand blockchain-tracked component sourcing (e.g., via VeChain) to combat counterfeit parts
- Tariff Engineering: Structure shipments under HTS 9018.41.00 (dental units) vs. 9018.49.00 for 3.5% duty savings
- Sustainability: 67% of US clinics now require carbon-neutral shipping (verify via EcoVadis score)
Note: All manufacturers must provide 2026 US Inflation Reduction Act (IRA) compliance documentation for clinic tax credit eligibility.
Final Recommendation: Prioritize manufacturers with US-based service networks. Carejoy’s partnership with Dental Tech Alliance ensures 48-hour US technician response – critical for minimizing clinic downtime in 2026’s competitive market.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Key Considerations for Dental Clinics & Distributors
Need a Quote for Chair Manufacturers In Usa?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


