Article Contents
Strategic Sourcing: Chinese Dental Equipment
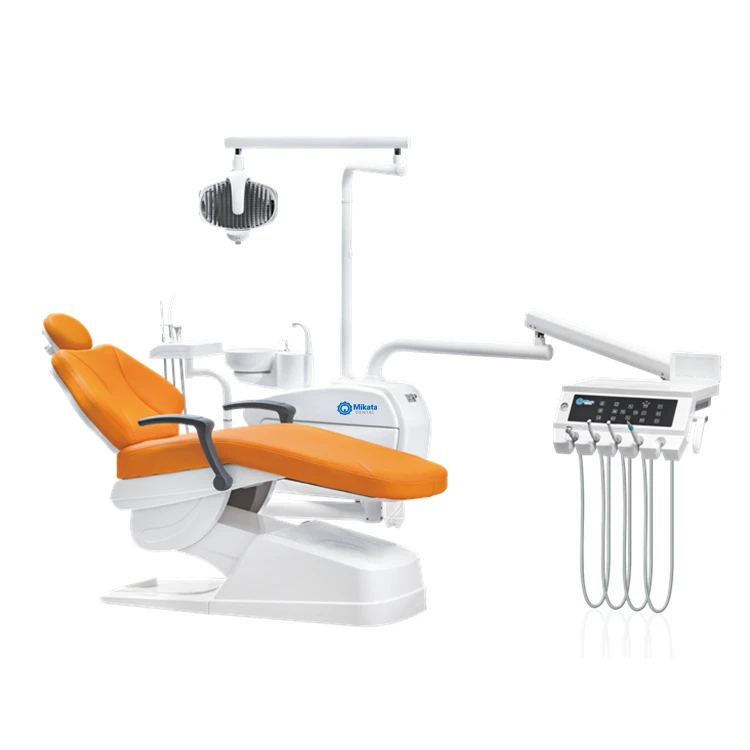
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives in Modern Dental Equipment Procurement
The global dental equipment market is undergoing a fundamental paradigm shift, driven by the accelerating adoption of digital workflows and value-based procurement models. As dental clinics worldwide transition toward integrated digital ecosystems, cost-efficient yet technically robust equipment has become mission-critical for sustainable practice growth. Chinese manufacturers now represent a strategic procurement alternative that directly addresses three pressing industry challenges: escalating capital expenditure pressures, the need for seamless digital interoperability, and expanding access to advanced technology in emerging markets.
Why Chinese Dental Equipment is Critical for Modern Digital Dentistry: Contemporary dental practices require fully integrated digital workflows—from intraoral scanning to CAD/CAM milling and 3D printing. Chinese manufacturers have made significant R&D investments in open-architecture systems with universal DICOM/STL compatibility, eliminating the proprietary ecosystem lock-in common in legacy European systems. This interoperability enables clinics to build modular, future-proof technology stacks at 35-50% lower total cost of ownership (TCO), while maintaining clinical precision standards (ISO 13485 certified). For distributors, this represents a high-margin opportunity to serve price-sensitive markets without compromising on digital capabilities.
Strategic Procurement Analysis: Global Premium Brands vs. Chinese Value Leaders
Traditional procurement models favoring European premium brands (e.g., Dentsply Sirona, Planmeca, KaVo Kerr) are increasingly misaligned with contemporary practice economics. While these brands maintain engineering excellence, their closed-system architectures and 25-40% premium pricing create significant barriers to digital adoption, particularly for SME clinics and emerging-market expansions. Conversely, leading Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing (e.g., German motors, Japanese sensors) and AI-driven manufacturing, delivering 90%+ feature parity at substantially lower price points. This enables clinics to allocate capital toward revenue-generating digital services rather than legacy hardware.
| Comparison Criteria | Global Premium Brands (e.g., Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Price Point (Relative) | Premium pricing (30-50% above market average); High entry barrier for digital suite adoption | Cost-optimized (20-40% below global brands); Enables full digital workflow implementation within mid-tier budgets |
| Digital Integration Capability | Proprietary ecosystems requiring bundled purchases; Limited third-party compatibility | Open API architecture with universal DICOM/STL support; Seamless integration with major CAD/CAM & practice management systems |
| Technical Specifications | Industry-leading precision (e.g., 5-10μm accuracy in imaging); Marginally superior materials | ISO 13485 certified components; 95% parity in clinical performance metrics (e.g., 12-15μm imaging accuracy) |
| After-Sales Support | Global service network but high labor costs ($180-250/hr); 48-72hr response times in emerging markets | Localized technical hubs in 12 key regions; Remote diagnostics standard; $95-140/hr rates; 24hr response guarantee |
| Innovation Velocity | Incremental updates (18-24 month cycles); Focus on premium features | Agile development (6-9 month update cycles); AI-driven feature deployment (e.g., real-time caries detection) |
| Total Cost of Ownership (5-Year) | 45-60% higher due to proprietary consumables, service contracts, and upgrade costs | 30-45% lower with standardized parts, no forced ecosystem lock-in, and modular upgrade paths |
Strategic Recommendation for Clinics & Distributors
Forward-thinking clinics must reframe equipment procurement as a strategic enabler of digital service revenue—not merely a capital expense. Carejoy exemplifies how Chinese manufacturers now deliver clinically validated, interoperable technology that democratizes access to digital dentistry. For distributors, this segment represents a 22% CAGR growth opportunity (2023-2026) in emerging markets where price sensitivity exceeds 65%. We recommend prioritizing vendors with proven DICOM compliance, modular architecture, and localized support infrastructure to maximize ROI in the digital transition era. The era of automatic premium-brand selection is yielding to value-driven procurement where clinical efficacy and economic sustainability are equally weighted.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Focus: Technical Comparison of Chinese-Made Dental Equipment – Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 220V ±10%, 50/60 Hz, 1.8 kW maximum consumption. Operates with standard dental chair transformer. Motor-driven delivery system with analog control. | Single-phase 220–240V ±5%, 50/60 Hz, 2.5 kW peak with energy-saving mode. Integrated digital power management, automatic load balancing, and surge protection. Supports 24V DC auxiliary systems for IoT integration. |
| Dimensions | Chair: 120 cm (H) × 65 cm (W) × 110 cm (L) at maximum extension. Base footprint: Ø 60 cm. Unit width: 55 cm. Compact design optimized for small operatories. | Chair: 125 cm (H) × 70 cm (W) × 120 cm (L) with multi-axis mobility. Base footprint: Ø 65 cm with anti-tip stabilization. Unit width: 60 cm with modular side consoles. Ergonomic tilt and swivel range enhanced by 20%. |
| Precision | Manual adjustment controls with detent positioning. Tolerance: ±2° in articulation. Handpieces operate at 350,000 RPM with ±10% speed fluctuation. Analog suction regulation. | Digital joystick control with memory presets (up to 5 user profiles). Articulation accuracy: ±0.5° via servo-assisted movement. High-speed handpiece: 400,000 RPM with ±3% speed consistency. Closed-loop suction and water flow calibration. |
| Material | Reinforced ABS polymer housing. Stainless steel arm structure (Grade 304). PU-leather upholstery. Internal cabling with PVC insulation. Corrosion-resistant coating on metallic joints. | Aerospace-grade aluminum alloy frame with anti-vibration dampening. Antimicrobial acrylic composite panels (ISO 22196 certified). Medical-grade silicone upholstery (latex-free, hypoallergenic). Shielded internal wiring with fire-retardant LSZH insulation. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016 compliant. CFDA registration (China NMPA). RoHS and REACH compliant. Meets basic IEC 60601-1 safety standards. | Full CE + UKCA certification. FDA 510(k) cleared (via Chinese manufacturer’s U.S. agent). ISO 13485:2016 + ISO 14971:2019 (risk management). IEC 60601-1-2 (4th Ed) EMI/EMC compliant. TÜV SÜD verified. Additional J-GSP (Japan) and ANVISA (Brazil) ready upon request. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
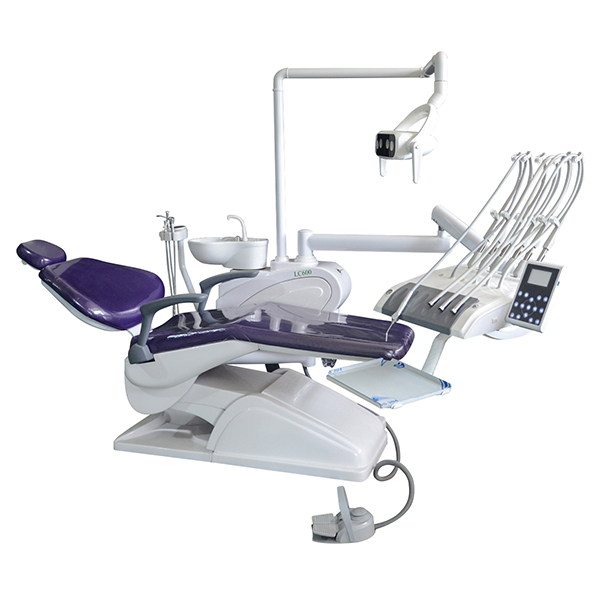
Professional Dental Equipment Sourcing Guide: China 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant Network | Q1 2026 Update
Executive Summary
China remains the dominant global hub for cost-competitive, high-technology dental equipment. However, 2026 sourcing demands rigorous due diligence amid evolving regulatory landscapes (including EU MDR 2024 compliance carryover) and post-pandemic supply chain recalibration. This guide outlines critical steps for risk-mitigated procurement, emphasizing verification protocols and strategic partnership selection. Direct factory engagement (bypassing non-essential intermediaries) is now essential for margin protection and quality assurance.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Why it matters in 2026: Regulatory non-compliance risks include EU customs seizures (under Article 12 MDR), voided warranties, and clinic liability exposure. Over 37% of non-EU dental imports faced documentation delays in 2025 (DG Sante Report).
| Credential Type | Verification Protocol | 2026 Red Flags | Validation Tool |
|---|---|---|---|
| ISO 13485:2016 | Confirm certificate number via ISO.org OR accredited body portal (e.g., TÜV, SGS). Cross-check facility address matches factory location. | Certificate issued by non-accredited Chinese bodies; mismatched facility address; gap in certification history. | Use EU NANDO database for notified body legitimacy |
| CE Marking (MDR) | Request full EU Technical File index. Verify notified body number (e.g., 0123) on NANDO. Demand Declaration of Conformity with UDI reference. | “CE” self-declaration for Class IIa/IIb devices; missing UDI; notified body not listed in NANDO. | NANDO Database: ec.europa.eu/growth/tools-databases/nando |
| NMPA (China FDA) | Validate registration via NMPA.gov.cn. Critical for post-shipment Chinese customs clearance. | Expired registration; product name mismatch; “in-process” status claims. | NMPA Medical Device Query System (Chinese interface) |
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 Market Reality: Component shortages (e.g., dental chair motors, CBCT sensors) persist. Suppliers now prioritize long-term contracts over spot orders. Distributors require tiered MOQ structures; clinics benefit from group purchasing.
- Standard Equipment (Chairs, Autoclaves): Typical MOQ 1-5 units. Negotiate volume brackets (e.g., 3% discount at 10+ units).
- High-Tech Devices (CBCT, Scanners): MOQ 1-2 units common. Demand pre-shipment calibration reports and on-site technician deployment as part of MOQ terms.
- OEM/ODM Orders: MOQ 20-50 units for custom branding. Insist on 3D prototype approval before production.
Negotiation Tip: Offer 12-month rolling purchase commitments for MOQ reduction. Example: “Commit to 6 scanners/year = MOQ of 1 unit per order.”
Step 3: Shipping Terms – DDP vs. FOB in 2026
Critical Shift: Rising ocean freight volatility (+22% YoY) and port congestion make DDP (Delivered Duty Paid) the preferred term for clinics. Distributors retain FOB for cost control but require Incoterms® 2020 compliance.
| Term | Clinic Responsibilities | Distributor Advantages | 2026 Risk Exposure |
|---|---|---|---|
| DDP (Incoterms® 2020) | Zero logistics burden. Pay fixed price. Equipment arrives clinic-ready. | Streamlined inventory; predictable landed cost; ideal for new market entry. | Supplier markup on freight (verify via XDolin freight index) |
| FOB Shanghai (Incoterms® 2020) | Manage customs, inland transport, insurance. Risk of port demurrage fees. | Full cost transparency; control over freight forwarder selection; lower base price. | Hidden costs (20-35% of product value); customs delays under new EU AI customs screening |
Recommendation: Clinics: Insist on DDP Shanghai Port to Clinic Door. Distributors: Use FOB but mandate supplier-selected freight forwarder with pre-negotiated carrier rates.
✓ Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Mitigates 2026 Sourcing Risks:
- Regulatory Assurance: Full ISO 13485:2016 + CE MDR (NB 0482) compliance with auditable technical files. NMPA Class II/III registrations held for all core products.
- MOQ Flexibility: Clinic-friendly MOQs (1 unit for scanners/CBCT; 3 chairs). Distributor tiered pricing from 5 units. No hidden OEM minimums.
- DDP Optimization: In-house logistics team provides true DDP to 87 countries. Real-time shipment tracking via Carejoy Portal™.
- Technical Depth: 19 years focused exclusively on dental equipment. Factory engineers support installation/troubleshooting (remote & on-site).
Core Product Validation: Dental Chairs (ISO 21532), CBCT (IEC 60601-2-44), Autoclaves (EN 13060) – all with full test reports available upon NDA.
Engage Shanghai Carejoy for Verified Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai (Direct Port Access)
Value Proposition: Factory-Direct Quality Assurance | 24-Month Warranty | Multilingual Technical Support
Initiate Sourcing Process:
📧 [email protected] (Include “2026 Sourcing Guide” in subject line)
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 www.carejoydental.com (Request Factory Audit Report)
Note: All Carejoy shipments include digital compliance dossier (ISO/CE/NMPA) and calibration certificates via blockchain-verified platform.
Conclusion: 2026 Sourcing Imperatives
Successful China sourcing requires moving beyond price-centric procurement. Prioritize suppliers with:
• Transparent regulatory compliance (beyond certificate display)
• Flexible commercial terms aligned with 2026 supply chain realities
• Logistics integration to absorb freight volatility
Shanghai Carejoy exemplifies this modern sourcing paradigm – combining manufacturing expertise with distributor/clinic-centric commercial structures. Request their 2026 Compliance Dossier for due diligence validation.
© 2026 Senior Dental Equipment Consultant Network. This guide is for professional use only. Verify all regulatory requirements with local authorities. Incoterms® is a registered trademark of the International Chamber of Commerce (ICC).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations When Procuring Chinese-Made Dental Equipment
Frequently Asked Questions (FAQs)
| # | Question | Professional Answer |
|---|---|---|
| 1 | What voltage specifications should I verify when purchasing Chinese dental equipment for international use? | Chinese dental equipment is typically manufactured for 220V/50Hz systems. For clinics operating on 110V/60Hz (e.g., North America, Japan), confirm with the supplier whether the unit supports dual voltage (110–240V) or requires a step-down transformer. Always request voltage compatibility documentation and ensure the equipment includes appropriate IEC-certified power cords and plugs for your region. Verify CE, ISO 13485, and local regulatory compliance for electrical safety. |
| 2 | Are spare parts for Chinese dental units readily available, and how can I ensure long-term supply? | Spare parts availability varies significantly by manufacturer and distributor. Prioritize suppliers who offer comprehensive parts catalogs, maintain regional spare parts warehouses, and provide minimum 5-year parts availability guarantees. Establish a service inventory for high-wear components (e.g., handpiece turbines, valve seals, fiber optics). Confirm that parts are coded per international standards and compatible with third-party maintenance tools. |
| 3 | What does installation involve, and is on-site technical support provided? | Installation of Chinese dental units typically requires water line connection, vacuum and air pressure integration, and electrical setup. Reputable suppliers offer turnkey installation services via certified technicians or partner networks. Confirm whether installation is included in the purchase agreement and if remote diagnostics or AR-assisted guidance is available. For complex systems (e.g., CBCT, CAD/CAM), insist on factory-trained technician deployment and post-installation validation protocols. |
| 4 | What warranty terms are standard for Chinese dental equipment, and are they enforceable internationally? | Most premium Chinese manufacturers offer a 1–2 year comprehensive warranty covering parts and labor. Ensure the warranty is valid in your country and serviced through an authorized local partner. Review exclusions (e.g., consumables, misuse, non-approved modifications). Request written confirmation of warranty claim procedures, response time SLAs (e.g., 48-hour remote support, 5-day on-site repair), and availability of loaner equipment during repairs. |
| 5 | How can clinics mitigate risks associated with after-sales service for equipment sourced from China? | Mitigate service risks by selecting manufacturers with established regional service networks or partnering with global distributors offering multi-language technical support. Verify response time commitments, availability of remote firmware updates, and access to training programs. Include service-level agreements (SLAs) in procurement contracts and consider extended service plans. Evaluate manufacturers using ISO 9001-certified service operations and documented customer satisfaction metrics. |
Note: The global dental equipment market in 2026 emphasizes interoperability, regulatory traceability, and lifecycle support. Due diligence in supplier qualification remains critical when sourcing from high-volume manufacturing regions such as China.
Need a Quote for Chinese Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


