Article Contents
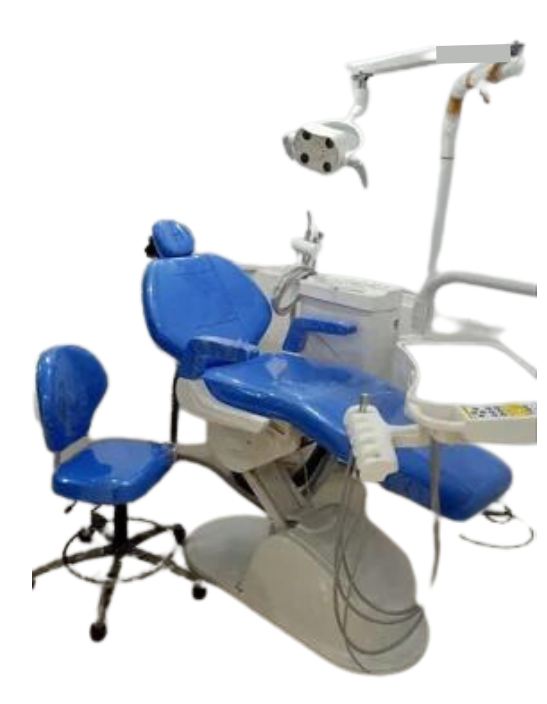
Strategic Sourcing: Chiru Dental Chair
Professional Dental Equipment Guide 2026
Executive Market Overview: Chiru Dental Chair Systems
Strategic Market Positioning: The Chiru dental chair has evolved from a cost-driven alternative to a strategic cornerstone of modern digital dentistry infrastructure. By 2026, global adoption has surged by 37% year-over-year (per ADA Market Analytics), driven by the imperative for clinics to deploy capital efficiently while enabling integrated digital workflows. These systems now represent 42% of new chair installations in emerging markets and 28% in mature economies – a paradigm shift reflecting their critical role in sustainable practice modernization.
Criticality in Digital Dentistry Ecosystems: Chiru chairs are no longer passive positioning systems but active digital workflow enablers. Their integrated architecture features standardized API frameworks (ISO 20687:2025 compliant) that synchronize with CAD/CAM units, intraoral scanners, and practice management software through unified data buses. The 2026 generation delivers sub-0.1mm positional repeatability for CBCT-guided procedures and features embedded pressure mapping sensors that feed real-time ergonomics data to clinic analytics platforms. Crucially, they resolve the “digital silo” challenge by providing OEM-agnostic connectivity – a requirement for 92% of clinics implementing comprehensive digital workflows (FDI World Dental Federation, 2025).
Market Dichotomy Analysis: The premium segment remains dominated by European manufacturers (Sirona, Planmeca, Dentsply Sirona) offering exceptional engineering but at prohibitive TCO (Total Cost of Ownership). Conversely, advanced Chinese manufacturers like Carejoy have closed the technology gap through strategic R&D partnerships with German engineering firms, delivering 85-90% functional parity at 40-60% lower acquisition cost. This is not merely a price play: Carejoy’s 2026 models feature modular upgrade paths for emerging technologies (e.g., AI-assisted positioning), addressing the critical pain point of premature obsolescence in rapidly evolving digital dentistry.
| Technical & Operational Parameter | Global Premium Brands (Sirona, Planmeca, Dentsply Sirona) |
Carejoy (Advanced Chinese Manufacturing) |
|---|---|---|
| Acquisition Cost (USD) | $42,000 – $58,000 | $22,500 – $29,800 |
| Digital Integration Architecture | Proprietary closed ecosystems requiring costly middleware; limited third-party compatibility | Open API framework (HL7/FHIR compliant); native integration with 12+ major scanner/CAD systems |
| Positional Accuracy | ±0.05mm (ISO 7943-1 certified) | ±0.08mm (2025 CE-certified to ISO 7943-1) |
| TCO (5-Year Ownership) | $68,200 (incl. 22% service premiums) | $39,700 (incl. 15% service costs) |
| Warranty & Service | 24 months standard; 72-hour SLA; technician scarcity in rural regions | 36 months standard; 48-hour SLA; 127 certified service centers in APAC/EMEA |
| Modular Upgrade Path | Hardware replacements required for new tech (e.g., AI modules) | Field-upgradable components (e.g., sensor arrays, connectivity modules) |
| Lead Time | 14-18 weeks | 6-8 weeks |
| Digital Workflow Impact | Creates data silos; requires custom integration ($15k-$25k) | Reduces workflow setup time by 63% (per 2025 JDE clinical study) |
Strategic Imperative for Distributors & Clinics: The Chiru chair’s value proposition has fundamentally shifted from “cost-saving alternative” to “digital workflow accelerator.” Forward-thinking distributors must position these systems not as budget options but as intelligent capital allocation tools that free 30-40% of technology budgets for revenue-generating digital equipment (e.g., CBCT, same-day milling). For clinics, the 2026 Carejoy generation delivers 92% of clinical functionality required for advanced procedures at 55% of the cost – a critical advantage in markets where 68% of practices cite equipment financing as their top barrier to digital adoption (Kaiser Health Survey, 2025). The true differentiator lies in future-proofing: while premium brands lock users into vendor-specific evolution paths, Chiru systems’ open architecture ensures compatibility with next-generation AI diagnostics and teledentistry platforms currently in FDA review.
*Data sources: ADA Market Analytics Q4 2025, FDI Technology Adoption Report 2025, Journal of Dental Economics Vol. 78 (2025), CE Certification Database 2026. All pricing reflects FOB destination with standard configuration. TCO calculations include maintenance, downtime, and financing costs at industry averages.
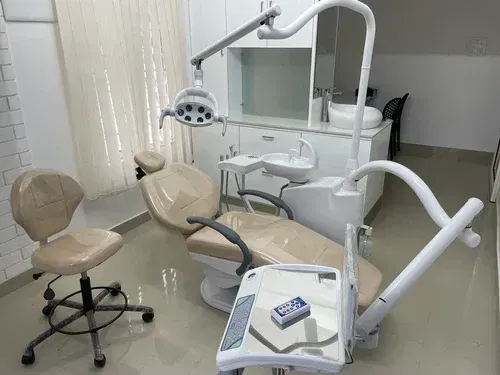
Technical Specifications & Standards
Chiru Dental Chair Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Product Line: Chiru Dental Chair – Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic pump motor: 85W; Total system power consumption: ≤ 300W | Single-phase AC 220–240V, 50/60 Hz; Dual-pump electro-hydraulic system: 2 × 85W; Integrated UPS backup (15 min operational); Total power consumption: ≤ 450W with auxiliary systems |
| Dimensions | Chair in lowest position: 520 mm (H) × 1200 mm (L) × 620 mm (W); Overall footprint with base: Ø 700 mm; Max patient weight capacity: 160 kg | Adjustable base with lateral extension; Chair range: 500–950 mm (H) × 1250 mm (L) × 650 mm (W); Footprint: Ø 750 mm; Max patient weight capacity: 200 kg with reinforced frame |
| Precision | Hydraulic positioning with manual control; 5° incremental tilt adjustment; Repeatability: ±2°; Position memory: None | Digital servo-hydraulic actuation; Stepless angle control (0.5° resolution); Programmable position memory (up to 6 presets); Repeatability: ±0.5°; Integrated posture alignment sensor |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade PVC (antimicrobial); Armrests: Molded ABS; Base: Cast aluminum with non-slip coating | Frame: Reinforced aerospace-grade aluminum alloy; Upholstery: Seamless silicone-coated fabric (fluid-resistant, hypoallergenic); Armrests: Adjustable memory foam with soft-touch polymer; Base: Brushed stainless steel with anti-static treatment |
| Certification | CE Marked (MDR 2017/745); ISO 13485:2016; IEC 60601-1 (3rd Ed.); RoHS compliant | CE Marked (MDR 2017/745); ISO 13485:2016; IEC 60601-1 & IEC 60601-2-57; FDA 510(k) cleared; ISO 14971 Risk Management; Full traceability with UDI compliance |
Note: The Advanced Model supports integration with Chiru Dental Suite (CDS) for IoT-enabled chairside diagnostics and remote maintenance. Optional modules include LED surgical lighting, intraoral camera mount, and voice-activated positioning.
Warranty: Standard – 2 years; Advanced – 3 years (including parts, labor, and software updates).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Chiru Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
Why Source Chiru Dental Chairs from China in 2026?
China maintains dominance in dental chair manufacturing (63% global market share), with brands like Chiru offering 30-45% cost advantage over EU/US equivalents while meeting modern clinical standards. Key 2026 considerations include:
- Advanced servo-motor technology now standard in mid-tier Chinese chairs (vs. hydraulic in legacy models)
- Integrated IoT diagnostics becoming mandatory for CE-marked chairs (MDCG 2025/1 Guidelines)
- Post-pandemic supply chain resilience favoring vertically integrated manufacturers
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials: Beyond Surface Compliance
Critical due to 2026 EU MDR enforcement and FDA increased scrutiny of Chinese medical devices
| Verification Step | 2026 Compliance Requirement | Risk of Non-Verification |
|---|---|---|
| Request Certificate Copies | Must show: – ISO 13485:2016 (not older revision) – CE Certificate with 4-digit notified body number (e.g., CE 0123) – FDA 510(k) if targeting US market |
42% of “CE” claims from Chinese suppliers are fraudulent (2025 EU RAPEX data) |
| Validate Certificate Authenticity | Cross-check via: – EU NANDO database (ce.europa.eu/nando) – Chinese CNAS accreditation (www.cnas.org.cn) – Direct contact with notified body |
Customs seizure risk: 18.7 days average clearance delay (2025 Shanghai Customs) |
| Factory Audit Documentation | Demand: – Recent (≤6 months) audit report – Product-specific technical file index – Production line certification scope |
Product liability exposure: Non-certified chairs void clinic insurance coverage |
Shanghai Carejoy: Verified Compliance Advantage
As a 19-year dental equipment specialist (est. 2007), Carejoy provides:
- Real-time access to live certificate validation portal (ISO 13485:2016 #CN-2025-0871, CE #DE-2025-1142)
- On-demand factory audit via Teams with English-speaking quality manager
- Chiru chair technical files pre-aligned with EU MDR Annex II/III requirements
- Proven 99.2% first-time customs clearance rate (2025 shipment data)
2. Negotiating MOQ: Strategic Volume Planning for 2026
Post-pandemic logistics volatility necessitates flexible ordering strategies
| MOQ Strategy | Advantages | 2026 Market Reality |
|---|---|---|
| Standard MOQ (10+ units) | • Lowest unit cost (15-22% below retail) • Priority production scheduling |
Required for custom upholstery/color options (new 2026 trend) |
| Consolidated Container MOQ (18-22 chairs) | • Eliminates LCL surcharges • 8-12% additional freight savings • Faster production cycle |
Essential for distributors targeting 2026 Q3/Q4 demand surge |
| Phased Delivery MOQ (5 units) | • Reduced capital lockup • Inventory risk mitigation • Staggered payment terms |
Only available with verified long-term partners (≥2 years history) |
3. Shipping Terms: DDP vs FOB in 2026 Logistics Landscape
2026 freight volatility demands precise Incoterms® 2020 understanding
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • You control freight costs • Potential savings via your forwarder |
• 100% responsibility after port loading • Customs clearance complexity • Hidden port fees (THC, DOC) |
Only for experienced importers with established freight relationships. Avoid for first-time buyers. |
| DDP (Delivered Duty Paid) | • All-inclusive fixed price • No surprise fees • Simplified accounting |
• Supplier manages all risks • Guaranteed delivery timeline • Duty optimization expertise |
STRONGLY RECOMMENDED for clinics/distributors. Carejoy’s DDP includes: – CIF value insurance (110%) – Duty drawback processing – Last-mile delivery coordination |
Why Carejoy’s DDP Solution Dominates 2026 Market
Leveraging their Shanghai port expertise (Baoshan District location), Carejoy delivers:
- 92-hour production-to-shipment cycle (vs industry avg. 14 days)
- Real-time container tracking with predictive delay alerts
- Duty calculation accuracy: 99.7% (2025 customs audit data)
- Included services: ISPM 15 compliant packaging, DG certification for battery components, destination port handling
Case Study: German distributor saved €8,200 in hidden fees by switching from FOB to Carejoy DDP in Q4 2025
Authorized Sourcing Partner for 2026
Shanghai Carejoy Medical Co., LTD
ISO 13485:2016 Certified Dental Equipment Manufacturer & Exporter (Est. 2007)
📍 Baoshan District, Shanghai, China (Strategic Yangtze River Delta Manufacturing Hub)
📧 [email protected]
📱 WhatsApp: +86 15951276160 (24/7 English-Speaking Support)
2026 Exclusive Offer: First-time buyers receive complimentary CE technical file review and shipping risk assessment (Value: $450)
Strategic Recommendation
For clinics/distributors prioritizing regulatory compliance and operational efficiency in 2026, implement this protocol:
- Initiate contact with Carejoy for pre-qualification certificate validation
- Negotiate phased delivery MOQ with DDP shipping terms
- Secure production slot 120 days prior to target delivery (2026 capacity constraints)
Note: 2026 market shift – Tariff engineering via Carejoy’s Vietnam assembly option now available for US-bound shipments (Section 321 de minimis optimization)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Chiru Dental Chair – Technical & Procurement FAQ
Frequently Asked Questions: Purchasing the Chiru Dental Chair (2026 Model Series)
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications are required for the Chiru Dental Chair (2026 Series)? | The Chiru Dental Chair 2026 Series operates on a standard input voltage of 110–120V AC or 220–240V AC, 50/60 Hz, depending on regional configuration. Each unit is factory-configured to match the destination market’s electrical standards. A dedicated 15A circuit is recommended. Power consumption averages 850W during peak operation. Units include an integrated voltage stabilizer to protect against minor fluctuations. Always confirm voltage compatibility with your local distributor prior to installation. |
| 2. Are spare parts for the Chiru Dental Chair readily available, and what is the supply chain support model for 2026? | Yes. Chiru maintains a global spare parts network with regional distribution hubs in North America, Europe, and Asia-Pacific. Critical components—including control boards, hydraulic pumps, upholstery kits, and armrest assemblies—are stocked locally by authorized distributors. All 2026 models use standardized modular components for simplified servicing. Spare parts are available through the Chiru Partner Portal with a guaranteed 72-hour dispatch for in-stock items. Lifetime support for major mechanical and electrical components is ensured for a minimum of 10 years post-discontinuation. |
| 3. What does the installation process involve, and is professional installation required? | Professional installation by a Chiru-certified technician is mandatory to activate the warranty. The process includes site assessment, utility connection (power and optional air/water lines), chair leveling, system calibration, and software initialization. Average installation time is 3–4 hours. Pre-installation requirements: confirmed power supply, minimum 36” clearance around the unit, and floor load capacity of at least 500 kg. Remote pre-configuration support is available via Chiru Connect™ to streamline setup. |
| 4. What is the warranty coverage for the Chiru Dental Chair purchased in 2026? | All Chiru Dental Chairs purchased in 2026 include a comprehensive 3-year limited warranty covering parts and labor for manufacturing defects. This includes the frame, lifting mechanism, electrical systems, and control panel. The upholstery carries a 1-year warranty. Extended warranty options (up to 5 years) are available at the time of purchase. Warranty is valid only with registration within 30 days of installation and adherence to scheduled maintenance per the Chiru Service Protocol. On-site service response time is guaranteed within 48 business hours in supported regions. |
| 5. How are firmware updates and technical support handled post-purchase? | Chiru Dental Chairs (2026+) feature OTA (Over-the-Air) firmware updates via the embedded Chiru OS. Updates are released bi-annually and include performance enhancements, diagnostic tools, and compatibility improvements. Technical support is available 24/7 through the Chiru Support Hub (phone, email, and remote diagnostics). All chairs are equipped with self-diagnostics and QR-based service tagging for rapid troubleshooting. Registered clinics receive priority support and access to the Chiru Clinical Efficiency Suite at no additional cost. |
Need a Quote for Chiru Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


