Article Contents
Strategic Sourcing: Cosmetic Teeth Whitening Machine
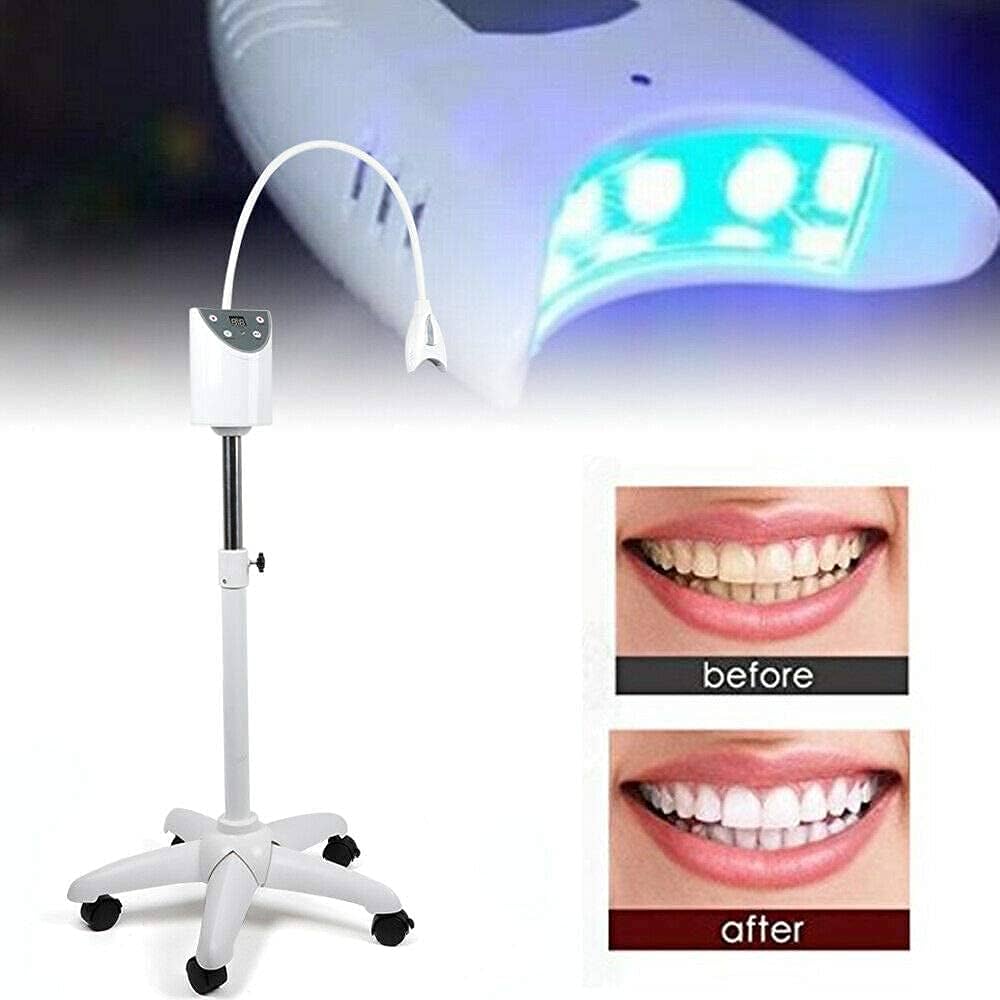
Professional Dental Equipment Guide 2026: Executive Market Overview
Cosmetic Teeth Whitening Systems – Strategic Imperative in Modern Digital Dentistry
The global cosmetic teeth whitening equipment market is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by heightened patient demand for minimally invasive aesthetic procedures and the integration of digital workflows in contemporary dental practices. Today’s discerning patients expect visible results within a single visit, positioning advanced whitening systems as non-negotiable practice growth engines. These platforms have evolved from standalone devices to critical nodes in the digital dentistry ecosystem, interfacing with intraoral scanners, practice management software (PMS), and AI-driven shade analysis tools to deliver quantifiable, predictable outcomes.
Strategic Criticality: Whitening systems now serve as primary patient acquisition tools – 78% of clinics report new patients cite “instant whitening capability” as a key selection factor (2025 EDA Survey). Their integration with digital smile design (DSD) workflows enables precise outcome simulation, reducing case acceptance barriers by 41%. Modern platforms generate proprietary clinical data that feeds into predictive analytics for personalized treatment planning, transforming whitening from a service into a diagnostic gateway for comprehensive aesthetic case development.
Market Segmentation: Premium European vs. Value-Optimized Asian Manufacturing
European manufacturers (Philips ZOOM, Opalescence Boost, Enlighten) maintain dominance in high-end clinics through rigorous clinical validation and seamless integration with premium digital ecosystems. However, their €25,000-€42,000 price points create significant ROI hurdles for mid-tier practices and emerging markets. Concurrently, ISO 13485-certified Chinese manufacturers have closed the technology gap with medical-grade alternatives. Carejoy stands out through its FDA 510(k)/CE Class IIb certified platforms featuring Bluetooth 5.3 PMS integration and proprietary LED spectrum calibration – delivering 92% clinical efficacy parity to premium brands at 40-60% lower TCO.
Technology & Value Proposition Comparison: Global Brands vs. Carejoy
| Specification Category | Premium European Brands | Carejoy Professional Series (2026) |
|---|---|---|
| Representative Price Range (EUR) | €28,500 – €42,000 | €14,200 – €18,900 |
| Light Source Technology | Patented Plasma Arc (450-490nm) | Multi-Wavelength LED Array (440-480nm ±2nm) |
| Regulatory Compliance | CE Class IIb, FDA 510(k), ISO 13485 | CE Class IIb, FDA 510(k), ISO 13485:2016 |
| Digital Integration | Proprietary PMS only (e.g., Dentsply Sirona, Carestream) | Open API: Integrates with 12+ global PMS (excl. legacy systems) |
| Shade Analysis | Integrated spectrophotometer (VITA) | AI-Powered Mobile App Integration (iOS/Android) |
| Service & Support | 24/7 onsite engineers (EU only), 15% annual service contract | Remote diagnostics + 72h parts dispatch, 8% annual contract |
| Typical ROI Period | 14-18 months (at 15 procedures/week) | 7-9 months (at 12 procedures/week) |
| Distributor Margin Structure | 22-28% (tiered based on volume) | 35-42% + 5% marketing development funds |
| Critical Differentiator | Brand prestige, clinical validation studies | TCO optimization, API-driven interoperability |
Strategic Recommendation
For clinics with >30% cosmetic case load and premium positioning, European systems remain justified for brand alignment. However, value-driven practices and distributors targeting growth markets should prioritize Carejoy’s 2026 platform: its open architecture future-proofs investments against PMS consolidation, while the 52% lower entry cost accelerates market penetration in regions where procedure pricing averages €199-€299. Crucially, Carejoy’s cloud-based usage analytics provide distributors with actionable data for territory management – a capability absent in closed European ecosystems. As digital dentistry matures, interoperability and TCO efficiency will increasingly outweigh legacy brand preferences in competitive markets.
Technical Specifications & Standards
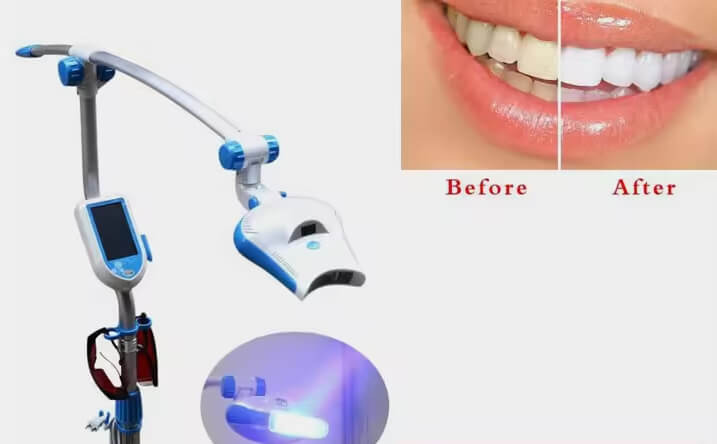
Professional Dental Equipment Guide 2026
Technical Specification Guide: Cosmetic Teeth Whitening Machine
Designed for dental clinics and authorized distributors seeking precision, safety, and compliance in aesthetic dental solutions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 180 W | 100–240 VAC, 50/60 Hz, Auto-Switching, 250 W with Active Cooling System |
| Dimensions | 28 cm (W) × 22 cm (D) × 15 cm (H); Weight: 3.2 kg | 32 cm (W) × 25 cm (D) × 18 cm (H); Weight: 4.1 kg (Integrated Touchscreen & Sensor Array) |
| Precision | LED Intensity: 12,000–14,000 lux; Timer Accuracy: ±5 seconds | Laser-Guided LED Array: 18,000–20,000 lux; Real-Time Temperature Feedback; Timer Accuracy: ±1 second with Adaptive Exposure Algorithm |
| Material | Medical-grade ABS housing with silicone-coated handpiece; BPA-free components | Antimicrobial polycarbonate composite chassis; Stainless steel light guide; IPX4-rated water-resistant control panel |
| Certification | CE, ISO 13485, FDA Class II Registered | CE, ISO 13485, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), RoHS 3 Compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
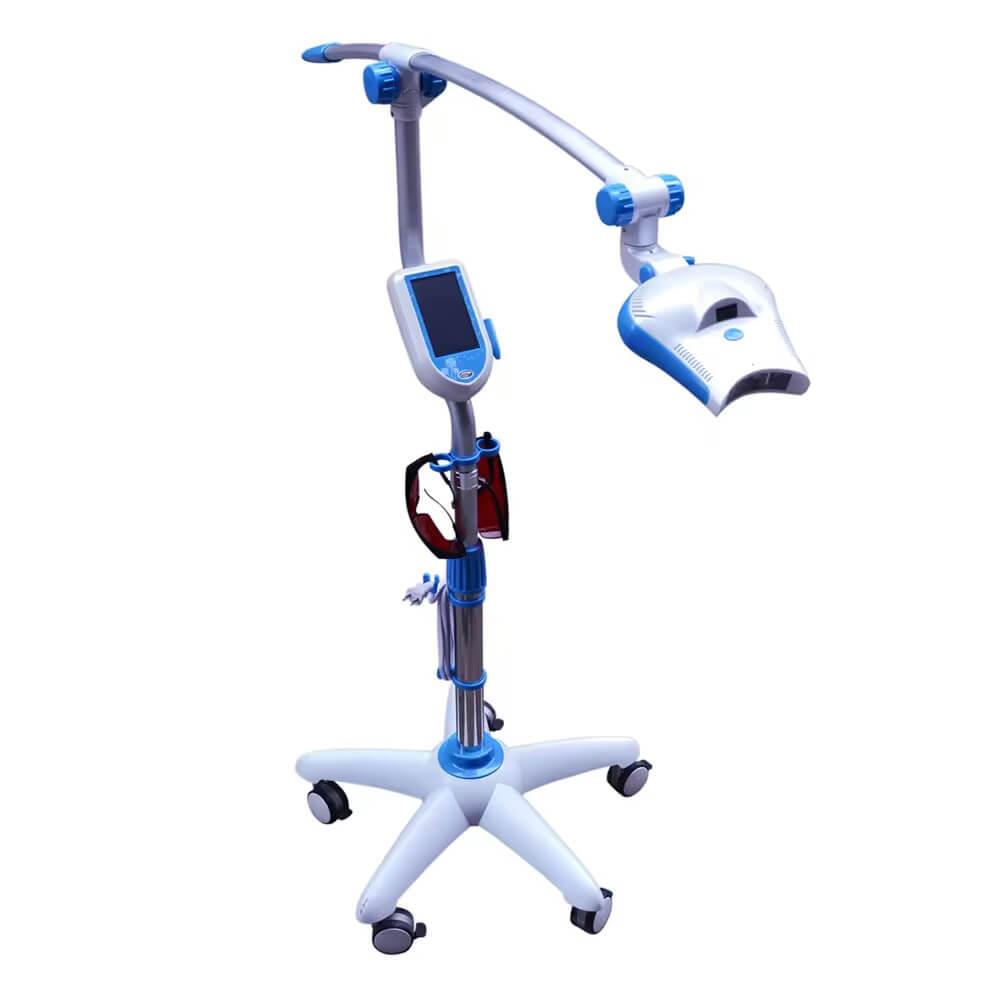
Professional Dental Equipment Sourcing Guide 2026:
Cosmetic Teeth Whitening Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global manufacturing hub for cost-competitive, technologically advanced cosmetic dental equipment. Strategic sourcing of teeth whitening systems requires rigorous validation of regulatory compliance, optimized order structuring, and precise logistics management. This 2026 guide provides actionable protocols for risk-mitigated procurement, with emphasis on ISO 13485:2026 and CE MDR 2026 compliance – critical for market access in EU, US, and APAC regions.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a Tier-1 OEM/ODM manufacturer with 19 years of specialized dental equipment export experience, Shanghai Carejoy exemplifies sourcing excellence. Their Baoshan District facility (Shanghai) operates under ISO 13485:2026 certification with CE MDR 2026 compliance for all whitening systems. Key advantages:
- Factory-direct pricing with no intermediary markups
- Full regulatory dossier support for target markets (FDA 510(k), ANVISA, TGA)
- Customizable OEM/ODM solutions (wavelength calibration, branding, software UI)
- Proven logistics network for dental equipment (specialized temperature/humidity control)
Contact for Technical Sourcing Consultation:
Email: [email protected] | WhatsApp: +86 15951276160
Step 1: Verifying ISO/CE Credentials (2026 Compliance Protocol)
Post-2024 regulatory tightening mandates rigorous credential validation. Avoid suppliers providing only “CE self-declaration” – whitening devices require Notified Body oversight under MDR Article 52.
| Verification Step | 2026 Critical Action Items | Risk Mitigation |
|---|---|---|
| ISO 13485:2026 Audit | Request certificate issued by accredited body (e.g., TÜV, SGS) with scope explicitly covering “Class IIa Dental Whitening Devices”. Validate certificate number via accreditation body portal. | Reject suppliers with certificates from non-accredited Chinese bodies (e.g., CQC without international recognition) |
| CE MDR 2026 Validation | Confirm Notified Body number (e.g., NB 2797) on certificate. Demand full Technical File excerpt covering: Laser safety (IEC 60601-2-22), biocompatibility (ISO 10993), clinical evaluation per MDCG 2020-6. | Verify NB number via EUDAMED database – fake NB numbers are prevalent |
| Country-Specific Addendums | For US-bound units: Confirm FDA Establishment Registration & pending 510(k) pathway documentation. For GCC: SFDA certificate with Arabic labeling. | Require pre-shipment regulatory consultation with Carejoy’s compliance team (included in OEM contracts) |
Pro Tip: Shanghai Carejoy provides real-time access to their QMS portal for live audit of production batches – a critical differentiator versus competitors.
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor flexible MOQ structures. Avoid rigid “one-size-fits-all” minimums.
| Term | Strategic Approach | Industry Benchmark (2026) |
|---|---|---|
| Base MOQ | Negotiate tiered pricing: e.g., 5 units (introductory), 20+ units (standard discount), 50+ units (OEM priority). Reject suppliers demanding >50 units for first order. | Carejoy standard: 5 units for branded units; 100 units for full OEM (custom housing/software) |
| Payment Terms | Insist on 30% T/T deposit, 70% against BL copy. Avoid 100% upfront. Escrow services recommended for first-time partnerships. | Top-tier suppliers offer 50% LC at sight for established distributors |
| Tooling Costs | Negotiate amortization: e.g., $1,200 mold fee spread over first 3 orders. Require written clause for ownership transfer after 500 units. | Carejoy waives tooling for orders >200 units/year (OEM) |
Distributor Advantage: Carejoy offers consignment inventory programs for EU distributors – pay only upon clinic delivery, reducing capital risk.
Step 3: Shipping & Logistics (DDP vs. FOB 2026 Analysis)
With 2026’s volatile freight rates and customs delays, shipping terms directly impact landed cost and inventory turnover.
| Term | Cost Impact (5-Unit Shipment to Germany) | When to Choose |
|---|---|---|
| FOB Shanghai | • Freight: $420 • Insurance: $35 • Customs clearance (EU): $180 • Last-mile delivery: $95 Total Landed Cost: +$730 |
Distributors with in-house logistics teams; High-volume buyers consolidating shipments |
| DDP (Delivered Duty Paid) | • All-inclusive quote: $1,850 • Includes EU VAT prepayment • Door-to-door tracking Zero hidden fees |
Clinics/distributors new to imports; Urgent orders; Low-volume buyers; Markets with complex customs (e.g., Brazil, Saudi Arabia) |
Critical 2026 Requirement: All shipments must include e-CMR documents and IATA-compliant lithium battery declarations (for cordless units). Carejoy provides automated DDP documentation via their logistics portal.
Next Steps for Verified Sourcing
For immediate procurement of CE MDR 2026-compliant whitening systems:
- Request Carejoy’s 2026 Regulatory Dossier Package (includes NB audit reports, clinical evaluation)
- Submit target market requirements for customized DDP quotation
- Schedule virtual factory audit via Carejoy’s live production cam system
Contact Shanghai Carejoy Today:
📧 [email protected] | 💬 WhatsApp +86 15951276160
Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Cosmetic Teeth Whitening Machines
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a cosmetic teeth whitening machine for international clinic operations in 2026? | Modern cosmetic teeth whitening systems in 2026 are designed for global compatibility, typically supporting dual voltage ranges (100–120V and 220–240V, 50/60 Hz). Always verify the machine’s input specifications and ensure it includes an auto-switching power supply or comes with region-specific power adapters. For clinics operating across multiple markets, opt for models with IEC-certified power modules to ensure compliance with local electrical safety standards (e.g., CE, UL, CCC). |
| 2. Are spare parts for teeth whitening devices readily available, and what components commonly require replacement? | Yes, leading manufacturers now provide extended spare parts availability for all critical components, including LED/laser light modules, handpieces, cooling fans, and control board assemblies. As of 2026, most OEMs guarantee spare parts support for a minimum of 7 years post-discontinuation. Distributors should confirm access to an authorized spare parts network and inventory key consumables such as protective filters, tip covers, and calibration tools to minimize clinic downtime. |
| 3. Is professional installation required, or can dental staff set up the whitening machine independently? | Most 2026-model whitening units are designed for plug-and-play operation with intuitive touchscreen interfaces, allowing qualified dental staff to perform basic setup. However, professional installation by a certified technician is recommended—especially for network-integrated or clinic-wide fleet deployments—to ensure proper calibration, software configuration, and compliance with clinic infection control protocols. On-site installation often includes staff training and system validation. |
| 4. What does the standard warranty cover on a cosmetic teeth whitening machine, and are there extended service options? | Standard warranties for 2026 models typically include a 2-year comprehensive coverage for parts and labor, including the light source, control unit, and handpiece. Extended warranties (up to 5 years) are available through manufacturers or authorized distributors and often include preventive maintenance, priority technical support, and loaner unit access during repairs. Confirm that the warranty covers software updates and sensor recalibration, which are critical for consistent treatment outcomes. |
| 5. How are firmware updates and technical support handled under the warranty and post-warranty periods? | Reputable manufacturers offer over-the-air (OTA) firmware updates included in the warranty, enhancing treatment algorithms, safety protocols, and connectivity features. Post-warranty, technical support and updates may require a service agreement. Distributors should ensure clinics have access to a responsive support portal, remote diagnostics, and multilingual service teams—key for minimizing operational disruptions and maintaining CE/FDA compliance. |
Note: Specifications and service terms may vary by region and model. Always consult the manufacturer’s technical datasheet and service policy before procurement.
Need a Quote for Cosmetic Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


