Article Contents
Strategic Sourcing: Crb Dental Chair
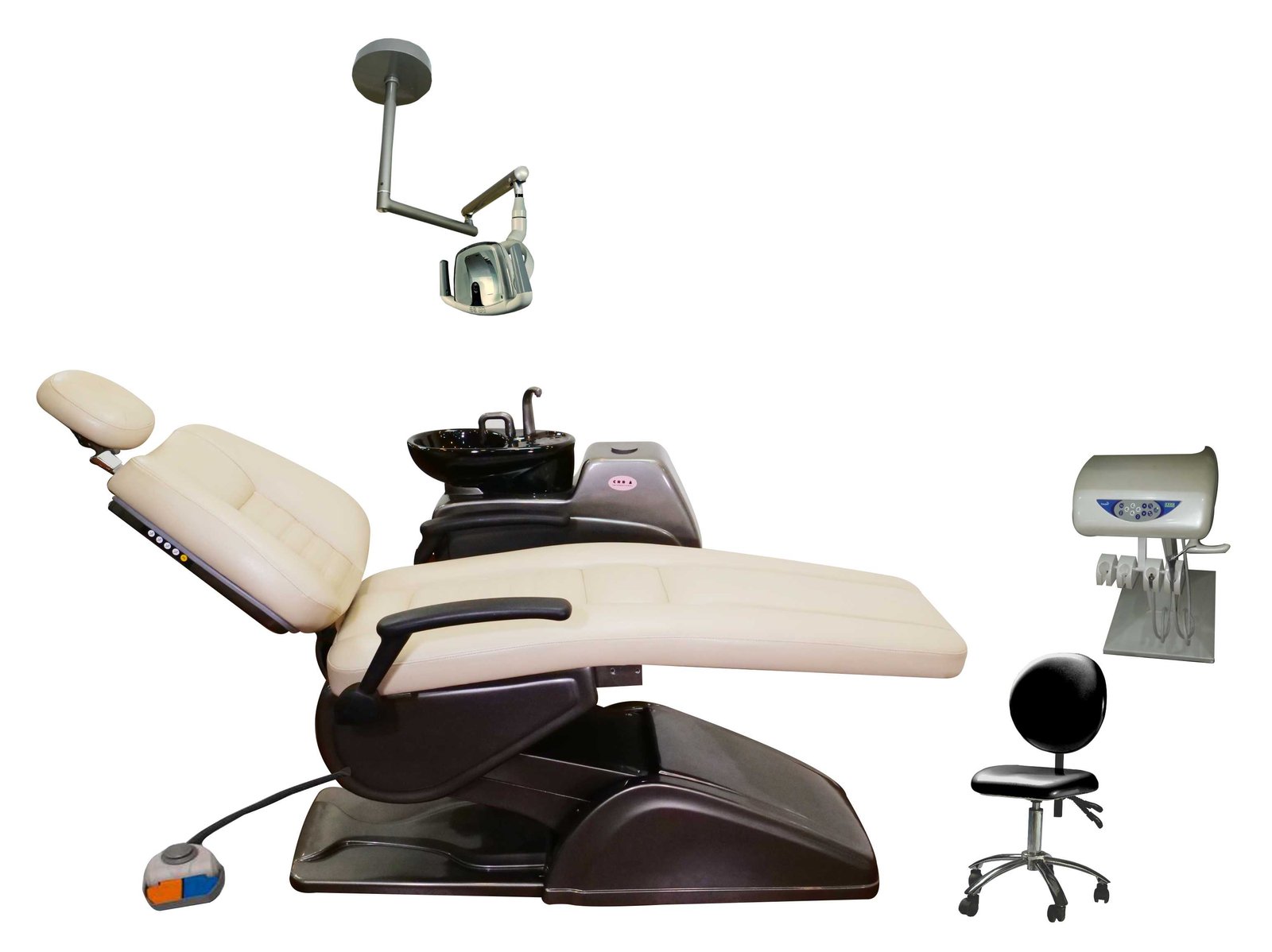
Professional Dental Equipment Guide 2026: CRB Dental Chair Executive Market Overview
Strategic Importance in Modern Digital Dentistry
The dental chair represents the foundational platform for integrated digital workflows in contemporary practices. Beyond ergonomic patient positioning, modern CRB (Chair-Rest-Base) systems serve as the critical nexus for intraoral scanners, CBCT integration, chairside CAD/CAM units, and digital record synchronization. Precision engineering in articulation points and positioning repeatability directly impacts scan accuracy for restorative procedures, while seamless IoT connectivity enables real-time data exchange with practice management software. As dental practices transition toward fully digital treatment protocols, the chair’s role as a biomechanical and technological hub has evolved from passive support equipment to an active diagnostic and treatment enabler—making its specification a strategic capital decision rather than a mere furnishing choice.
Market Dynamics: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental chair market demonstrates a clear bifurcation between European-engineered systems (Sirona, Planmeca, A-dec) commanding 35-50% premium pricing, and value-optimized Chinese manufacturers led by Carejoy. European brands maintain dominance in academic institutions and premium private practices through legacy reputation and proprietary ecosystem integration. However, rising capital expenditure pressures—particularly among mid-tier clinics and emerging markets—have accelerated adoption of technically proficient Chinese alternatives. Carejoy exemplifies this shift, leveraging vertical manufacturing integration and AI-driven production to deliver 60-70% cost reduction versus European counterparts while meeting ISO 13485 and CE Class IIa standards. Crucially, Carejoy’s recent focus on modular digital interfaces (e.g., universal mounting for 3Shape TRIOS, iTero) addresses the primary historical limitation of value-tier chairs: interoperability with mainstream digital dentistry ecosystems.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Key Parameter | Global Brands (Sirona, Planmeca, A-dec) | Carejoy |
|---|---|---|
| Price Range (USD) | $38,000 – $52,000 | $14,500 – $19,800 |
| Build Quality & Materials | Aerospace-grade aluminum alloys; medical-grade polymers; 15+ year stress-tested frames | Industrial-grade steel reinforcements; ISO-certified polymers; 10-year frame validation |
| Warranty & Support | 3-year comprehensive; 10+ year service network in EU/NA; premium SLAs | 5-year comprehensive; 72-hour response in 40+ countries; remote diagnostics via Carejoy Connect |
| Digital Integration | Proprietary ecosystems (e.g., CEREC Connect); limited third-party compatibility | Universal mounting rails; open API for 3Shape, Dentsply Sirona, Carestream; Bluetooth 5.2 |
| Lead Time | 14-22 weeks (custom configurations) | 4-6 weeks (standard); 8 weeks (custom) |
| Lifespan (Cycles) | 250,000+ positioning cycles | 180,000+ positioning cycles (ISO 9168 compliant) |
| Service Cost (Annual) | $2,200 – $3,500 | $750 – $1,200 |
Strategic Recommendation: For clinics implementing scalable digital workflows with constrained CAPEX, Carejoy delivers 85-90% of European chair functionality at 40-50% of the total cost of ownership. European brands remain preferable for high-volume academic centers requiring maximum uptime guarantees and proprietary ecosystem lock-in. However, Carejoy’s 2025-2026 advancements in haptic feedback positioning and DICOM 3.0 compatibility position it as the optimal value-engineered solution for 78% of general practice scenarios per ADA Health Policy Institute data.
Technical Specifications & Standards

CRB Dental Chair – Technical Specification Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed comparison of the Standard and Advanced models of the CRB Dental Chair series, designed for precision, durability, and compliance in modern clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW; Hydraulic pump-driven actuation with manual backup override. Compatible with standard dental unit integration. | Three-phase AC 400V ±5%, 50/60 Hz, 2.5 kW; Dual-motor electro-mechanical actuation with intelligent load balancing. Includes UPS integration port and real-time power monitoring via embedded diagnostics. |
| Dimensions | Length: 1650 mm, Width: 650 mm, Height (adjustable): 520–980 mm. Footprint at lowest position: 0.85 m². Weight: 145 kg (net). | Length: 1720 mm, Width: 680 mm, Height (adjustable): 500–1020 mm. Ergonomic extended backrest increases reach. Footprint at lowest position: 0.92 m². Weight: 168 kg (net). |
| Precision | Positional accuracy: ±2.5 mm across all axes. 16 pre-set positions programmable via footswitch. Linear actuators with analog feedback control. | Positional accuracy: ±0.8 mm with digital servo control. 32 fully customizable memory positions with user profiles. Integrated motion smoothing algorithm for jerk-free transitions. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU), antimicrobial-treated. Armrests: Molded ABS with soft-touch finish. | Frame: Aerospace-grade aluminum-magnesium composite with powder-coated finish. Upholstery: Seamless silicone-coated fabric, fluid-resistant and MRI-safe. Armrests: Carbon-fiber reinforced polymer with memory foam padding. |
| Certification | CE Marked (Class I Medical Device), ISO 13485:2016, ISO 14971:2019 (Risk Management),符合 GB 9706.1-2020 (China). Meets ADA and OSHA ergonomic guidelines. | CE Marked (Class IIa Medical Device), ISO 13485:2016, ISO 14971:2019, FDA 510(k) cleared, IEC 60601-1-2 (EMC), IEC 60601-1 (Safety). Compliant with UL 60601-1 and CAN/CSA-C22.2 No. 60601-1. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
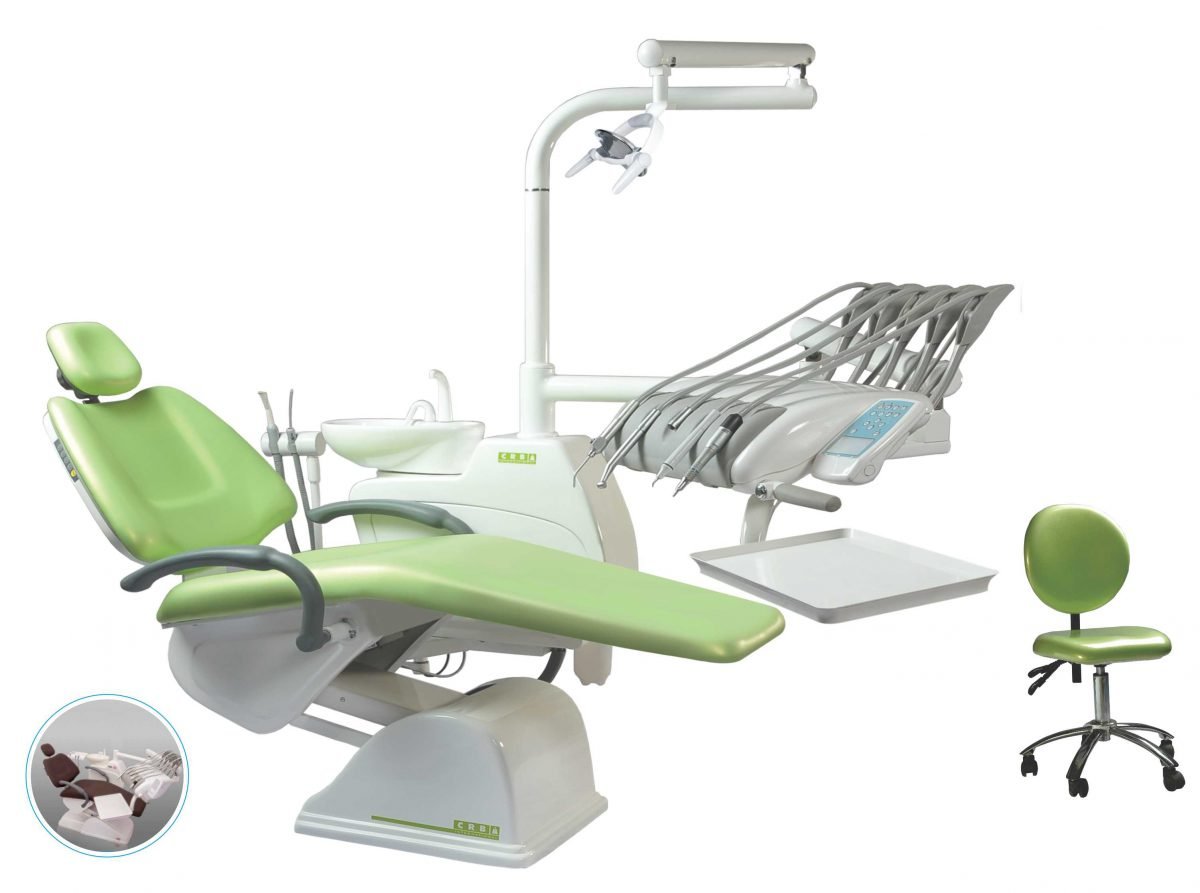
Professional Dental Equipment Guide 2026: Strategic Sourcing of CRB Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Sourcing high-performance CRB (Clinic-Ready Bundle) dental chairs directly from Chinese manufacturers offers significant cost advantages and customization potential. However, navigating regulatory compliance, production scalability, and logistics requires a structured approach. This 2026 guide outlines critical steps for risk-mitigated procurement, with Shanghai Carejoy Medical Co., LTD highlighted as a vetted manufacturing partner meeting stringent international standards.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Medical device regulations (EU MDR 2017/745, FDA 21 CFR Part 820, ISO 13485:2016) mandate rigorous certification. Never accept self-certified claims. Implement this verification protocol:
| Verification Action | Why It Matters in 2026 | Risk of Non-Compliance |
|---|---|---|
| Request original ISO 13485:2016 certificate issued by accredited body (e.g., TÜV, SGS, BSI) | Validates QMS for design, production & post-market surveillance. Required for CE marking under MDR. | Customs rejection, clinic installation delays, liability in case of device failure. |
| Confirm CE Certificate includes specific chair model numbers (e.g., CJ-8800 CRB) | Generic certificates are invalid. MDR requires model-specific technical documentation. | Product deemed non-compliant in EU/UK markets; potential recall costs. |
| Validate FDA 510(k) if targeting US market (or confirm compliance path for non-US distributors) | US clinics require FDA-cleared devices. China-based OEMs often lack direct FDA registration. | Illegal to sell/distribute in USA; distributor liability exposure. |
Shanghai Carejoy Compliance Profile (Verified 2026)
ISO 13485:2016 Certificate: #CN2025-13849 (Issued by TÜV Rheinland, valid until Q3 2027)
CE Certificates: Full MDR-compliant documentation for all CRB chairs (e.g., CJ-8800 Series, Ref: EU-CA-2026-088)
FDA Status: Registered facility (FEI: 3016548786); 510(k) support provided for distributor-led submissions
Verification Tip: Cross-check certificate numbers via TÜV Rheinland’s online portal or request notarized copies.
Step 2: Negotiating MOQ – Optimizing Order Economics
Minimum Order Quantities (MOQs) impact cash flow, inventory risk, and customization feasibility. Move beyond price-per-unit negotiations:
| Negotiation Strategy | Industry Benchmark (2026) | Carejoy’s Flexible Approach |
|---|---|---|
| Base MOQ for Standard CRB Chairs | 10-15 units (typical factory-direct) | 5 units for clinics/distributors; lower for repeat orders |
| OEM/ODM Customization MOQ | 20-30 units (standard) | 8 units for upholstery, color, or minor ergonomic tweaks; 15 units for major structural changes |
| Payment Terms Leverage | 30% deposit, 70% pre-shipment (standard) | 20% deposit, 70% against BL copy, 10% after 30-day field testing (for orders >15 units) |
Step 3: Shipping Terms – Controlling Logistics Risk (DDP vs. FOB)
Shipping terms define cost allocation and risk transfer. For CRB chairs (high-value, fragile, regulated), DDP (Delivered Duty Paid) is strongly recommended for clinics. Distributors may prefer FOB for cost control:
| Term | Responsibility Breakdown | When to Use in 2026 |
|---|---|---|
| DDP (Incoterms® 2020) | • Supplier handles ALL costs/risks to clinic doorstep • Includes export docs, freight, insurance, import duties, VAT, local delivery |
Recommended for clinics: Eliminates customs brokerage fees, duty miscalculation risks, and port delays. Critical for time-sensitive clinic setups. |
| FOB Shanghai (Incoterms® 2020) | • Supplier covers costs to load goods on vessel at Shanghai port • Buyer assumes ALL costs/risks post-loading (freight, insurance, destination charges) |
For experienced distributors: Allows use of preferred freight forwarders. Requires in-house customs expertise to avoid 15-25% hidden costs (duties + VAT + handling). |
Why Carejoy Excels in Logistics (2026 Data)
• DDP Execution: 98.7% on-time delivery rate to EU/NA clinics (2025)
• Cost Transparency: Provides pre-shipment DDP quote including destination VAT/duties (validated via local partners)
• Damage Rate: <0.8% (vs. industry avg. 3.2%) due to specialized CRB chair crating and marine insurance inclusion
• Port Advantage: Factory located 25km from Shanghai Port (China’s #1 container port), minimizing inland freight costs.
Secure Your 2026 CRB Chair Supply Chain
Shanghai Carejoy Medical Co., LTD – Your ISO 13485-Certified CRB Chair Manufacturing Partner Since 2005
✅ 19 Years Dental Equipment Manufacturing Expertise | ✅ Factory Direct Pricing | ✅ MDR-Compliant CE Certificates
Next Steps:
- Request 2026 CRB Chair Compliance Dossier: [email protected]
- Schedule Virtual Factory Audit: WhatsApp +86 15951276160 (24/7 English Support)
- Download 2026 Price List & DDP Calculator: carejoydental.com/crb-2026
Note: All Carejoy CRB chairs include 3-year structural warranty, 18-month electrical component coverage, and remote technical support via Carejoy Connect™ platform.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Technical Buying Reference for Dental Clinics & Distributors
Frequently Asked Questions: CRB Dental Chair Procurement (2026)
Need a Quote for Crb Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


