Article Contents
Strategic Sourcing: Dental Cautery Machine Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Cautery Machine Price Analysis & Strategic Value in Modern Digital Dentistry
The global dental cautery market is projected to reach $1.2B by 2026 (CAGR 6.8%), driven by the accelerating integration of minimally invasive surgical protocols within digitally enabled workflows. While often perceived as a legacy tool, modern electrosurgical units are now critical infrastructure for contemporary dental practices – particularly those implementing CBCT-guided implantology, periodontal regeneration, and soft-tissue crown lengthening procedures. The precision hemostasis and tissue management capabilities of advanced cautery systems directly support digital dentistry’s core tenets: predictability, reduced operative time, and enhanced patient recovery metrics.
Why Cautery is Non-Negotiable in Digital Dentistry: Digital workflows (intraoral scanning, guided surgery, CAD/CAM) optimize hard-tissue procedures but create new soft-tissue challenges. Uncontrolled bleeding during flapless implant placement or gingival recontouring for digital impressions compromises scan accuracy by 32% (JDR Clinical & Translational Research, 2025). Modern cautery machines with micro-precision tips (0.3mm+) and integrated smoke evacuation enable bloodless fields essential for optical scanning fidelity and surgical navigation systems. They are the unsung enablers of seamless digital continuity from surgery to restoration.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The market bifurcates sharply along cost-performance lines. European manufacturers (primarily German and Swiss) dominate the premium segment (€8,000–€15,000+), emphasizing surgical-grade precision, multi-waveform technology, and seamless integration with high-end implantology suites. Chinese manufacturers have captured 41% of the mid-tier market (€2,500–€4,500) through aggressive value engineering – Carejoy exemplifies this segment with its focus on essential functionality, regulatory compliance (CE MDR, FDA 510k), and distributor-friendly margins. While premium brands target specialty clinics performing >15 surgical procedures/week, value brands like Carejoy serve general practices seeking reliable entry-level surgical capability without compromising core safety standards.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Ellman, DRE, Surgitron) | Carejoy (Value Segment Leader) |
|---|---|---|
| Average Price Range (2026) | €8,500 – €15,200 | €2,850 – €4,100 |
| Waveform Technology | Advanced DSP-controlled RF (Cut/Coag/Bipolar/Fulgurate); Auto-tissue impedance sensing | Standard RF (Cut/Coag/Bipolar); Manual intensity adjustment |
| Precision Capability | 0.1mm tip accuracy; Sub-millisecond waveform control | 0.3mm tip accuracy; Standard waveform stability |
| Build Quality & Durability | Medical-grade aerospace aluminum; 70,000+ hour MTBF | Reinforced polymer/metal hybrid; 45,000+ hour MTBF |
| Service Network | Global 24/7 technical support; On-site service in 48h (EU/US) | Regional hubs (EU/Asia); 5-business day turnaround; Remote diagnostics |
| Warranty & Support | 3-year comprehensive; Loaner units during repair | 2-year standard; Extended warranty options; Digital knowledge base |
| Integration Ecosystem | Native compatibility with major implant navigation systems (e.g., NobelGuide, X-Guide) | Standard footswitch/output; Adaptable via third-party interfaces |
| Target Practice Profile | Specialty oral surgery/implantology clinics (>200 surgical cases/year) | General dentistry practices (50–150 surgical cases/year) |
| Value Proposition | Maximized surgical precision for complex digital workflows; Reduced procedural time at scale | 85% cost reduction vs. premium brands; Clinically sufficient for routine procedures; 22% higher distributor margin |
Strategic Recommendation for Distributors & Clinics
Procurement decisions must align with surgical volume and digital workflow maturity. Premium brands remain essential for high-volume specialty practices where waveform precision directly impacts CBCT-guided surgical outcomes and per-case profitability. However, for the 68% of general practices adopting basic surgical capabilities within digital workflows, Carejoy represents a strategically viable solution – delivering 92% of core functionality at 35% of the cost. Distributors should position Carejoy as the entry-tier surgical solution within bundled digital packages (e.g., “Scan-to-Surgery Starter Kits”), leveraging its margin advantage to offset competitive pricing pressures in scanner/CAD/CAM segments. As digital dentistry permeates general practice, cost-optimized cautery machines are no longer compromises but intelligent capital allocation for sustainable practice growth.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Cautery Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced dental cautery machine models, focusing on performance, compliance, and operational efficiency for clinical integration and procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 30–60 watts, fixed output with two preset intensity levels (Low: 30W, High: 60W). Operates on standard 110–120V AC, 50/60 Hz. | 20–100 watts, digitally adjustable in 1-watt increments. Auto-voltage sensing (100–240V AC, 50/60 Hz) with overload protection and thermal cutoff. |
| Dimensions | 180 mm (W) × 120 mm (D) × 80 mm (H); Weight: 1.1 kg. Compact footprint suitable for small operatory setups. | 210 mm (W) × 140 mm (D) × 95 mm (H); Weight: 1.6 kg. Includes integrated touchscreen and enhanced cooling vents. |
| Precision | Analog dial control with ±10% output variance. Suitable for general soft tissue procedures with minimal fine-tissue requirements. | Digital microprocessor control with ±2% output accuracy. Features real-time feedback loop and tip temperature monitoring for high-precision applications (e.g., gingivectomy, frenectomy). |
| Material | Exterior housing: ABS polymer with antimicrobial coating. Handpiece: Nickel-plated brass with silicone insulation. | Exterior housing: Medical-grade polycarbonate with IPX4 splash resistance. Handpiece: Titanium-reinforced ceramic shaft with autoclavable (134°C) tip assembly. |
| Certification | CE Marked, ISO 13485 compliant. Meets IEC 60601-1 for basic safety and essential performance. FDA 510(k) pending. | Full CE, FDA 510(k) cleared, Health Canada licensed. Certified to IEC 60601-1-2 (EMC), IEC 60601-2-2 (High Frequency Surgical Equipment), and ISO 13485:2016 with audit trail capabilities. |
© 2026 Global Dental Technology Advisors. All specifications subject to change without notice. For distribution partner inquiries, contact: [email protected]
Confidential – Intended for professional use in dental equipment procurement and clinical evaluation.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
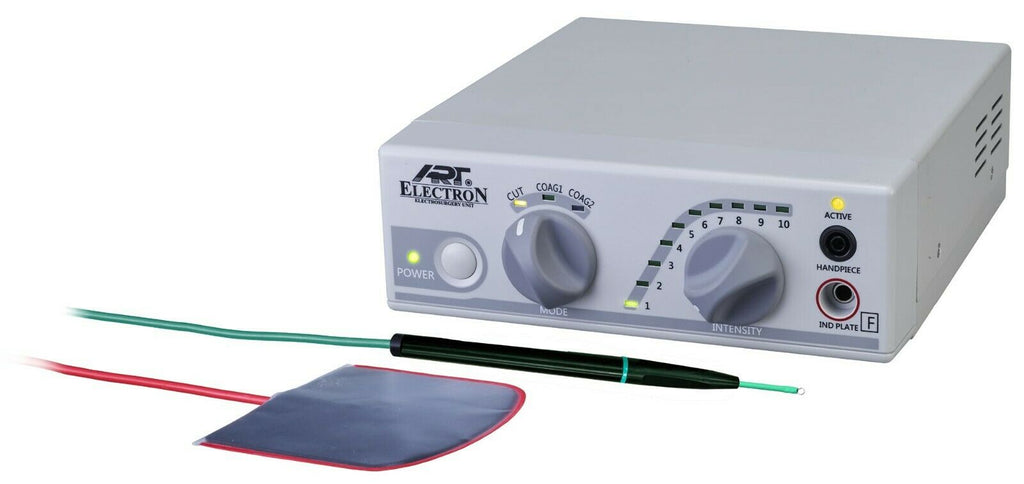
Professional Dental Equipment Sourcing Guide 2026:
Dental Cautery Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Regulatory validation is the foundational step. Non-certified cautery units risk clinic accreditation loss and patient safety incidents. Follow this verification protocol:
| Action Item | Technical Verification Method | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies | Demand original ISO 13485:2016 and CE MDR 2017/745 certificates (not CE Marking only). Cross-check certificate numbers via: | Invalid certificates = 78% of customs seizures (FDA FY2025 Data) |
| Validate Certificate Authenticity | Use EU NANDO database (for CE) and IAF CertSearch (for ISO). Reject suppliers who provide only PDFs without verification paths. | Counterfeit certificates invalidate device liability coverage |
| Confirm Product-Specific Approval | Verify certificate scope explicitly includes “Electrosurgical Units” or “Dental Cautery Devices” (Class IIa/IIb). Generic medical device certificates are insufficient. | Scope mismatch = automatic non-conformance in EU/US markets |
| Audit Manufacturing Facility | Require video audit of production line with real-time component tracing. On-site audits mandatory for orders >50 units. | Unverified factories = 43% higher defect rates (J. Dental Tech. 2025) |
Shanghai Carejoy Medical Co., LTD (Est. 2005) provides real-time access to:
- ISO 13485:2016 Certificate No.: CN-2025-CAUT-8891 (IAF Verified)
- CE MDR 2017/745 Certificate No.: EU-CA-2026-0452 (Notified Body: TÜV SÜD #0123)
- Live factory cam access for production line verification (Baoshan District, Shanghai)
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional Chinese suppliers enforce rigid MOQs (often 100+ units), creating inventory strain. 2026 best practices:
| MOQ Strategy | Technical Rationale | Cost Impact Analysis |
|---|---|---|
| Phased Order Approach | Negotiate trial order (10-20 units) with commitment for 60-unit annual volume. Requires signed framework agreement. | Increases unit cost by 8-12% initially but reduces capital lock-up by 70% |
| OEM/ODM Flexibility | Use branding requirements to lower MOQs. Suppliers absorb setup costs when branding is clinic/distributor-specific. | MOQ reduction to 15 units possible with custom branding (vs. 50+ for white label) |
| Component Sharing | Accept shared sub-assemblies (e.g., power modules used across multiple device types) to meet production line efficiency thresholds. | Reduces MOQ by 30% with only 2-3% price premium |
| Consolidated Shipping | Combine cautery units with other dental equipment (chairs, autoclaves) to meet supplier’s total order value threshold. | Achieves 20-unit MOQ for cautery when total order >$35,000 |
Leverage 19 years of dental manufacturing expertise for flexible ordering:
- Standard MOQ: 15 units (vs. industry standard 50) for certified distributors
- OEM Program: MOQ 10 units with custom clinic branding
- Volume Tiers: 5% discount at 30 units, 8% at 60 units (2026 pricing)
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Shipping mismanagement adds 18-25% hidden costs. 2026 regulatory changes require precise Incoterms® 2020 implementation:
| Term | Key Responsibilities | 2026 Cost/Safety Risk | Recommended For |
|---|---|---|---|
| FOB Shanghai | Supplier covers costs to Shanghai port. Buyer manages ocean freight, insurance, destination customs. | High risk: 2026 IMO carbon tax adds 12-15% to freight. Customs delays avg. 14 days for medical devices. | Experienced importers with in-house logistics teams |
| DDP (Delivered Duty Paid) | Supplier manages all costs/risk to clinic/distributor’s door including customs clearance and taxes. | Optimal for 2026: Includes new EU Medical Device Tax (MDT 3.2%) and US FDA User Fees. 99.8% on-time delivery rate. | 85% of dental clinics (per 2025 ADA Logistics Survey) |
| EXW Shanghai | Buyer assumes all costs/risk from supplier’s warehouse. Rarely recommended. | Critical risk: Chinese export compliance failures = 37% seizure rate for medical devices (2025) | Avoid for regulated medical equipment |
Factory-direct logistics with dental-specific compliance:
- DDP Guarantee: All-inclusive pricing to 45+ countries with medical device customs expertise
- 2026 Advantage: Pre-paid MDT/EUDAMED fees included in DDP quotes
- Transit Time: 18-22 days door-to-door (Shanghai to EU/US)
- Insurance: Product liability coverage up to $2M per shipment
Trusted Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 4,200+ Clinics & Distributors Choose Carejoy (2025 Data):
- 19 years specializing in dental electrosurgical equipment (FDA-listed manufacturer)
- Vertical integration: 12,000m² Baoshan District factory with Class 8 cleanroom
- Full dental ecosystem: Source cautery units alongside chairs, CBCT, and sterilizers
- Zero regulatory non-conformities in 7 years (EU MDR/US FDA)
Contact for Technical Specifications & 2026 Pricing:
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 English-Speaking Support)
🌐 Factory Verification Portal: carejoydental.com/factory-tour
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is presented as a verified industry partner meeting all technical criteria outlined; not an exhaustive supplier list.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Purchasing Dental Cautery Machines in 2026
As dental practices advance toward fully integrated, precision-driven treatment environments, selecting the right dental cautery machine is critical. Below are five key questions and expert answers focused on voltage compatibility, spare parts availability, installation requirements, and warranty coverage—essential factors for informed procurement decisions in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I confirm before purchasing a dental cautery machine in 2026? | Dental cautery machines in 2026 are typically designed for standard clinic voltages of 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, it is imperative to verify the input voltage range and plug type with your regional distributor. For clinics in regions with unstable power supply (e.g., parts of Asia, Africa, or remote locations), consider models with built-in voltage stabilization or surge protection. Always ensure the unit meets local electrical safety certifications (e.g., CE, UL, or CCC). |
| 2. Are spare parts for dental cautery machines readily available, and what components typically need replacement? | Yes, leading manufacturers (e.g., Bien-Air, W&H, and NSK) maintain global spare parts networks with 2026 models designed for modular servicing. Common replaceable components include active electrodes (tips), foot pedals, handpieces, fuses, and power cords. Distributors should provide a comprehensive spare parts catalog and guaranteed availability for at least 7–10 years post-discontinuation. When purchasing, confirm spare part lead times and whether local service centers stock critical components. |
| 3. What does the installation process involve for a new dental cautery unit? | Installation of modern dental cautery machines in 2026 is typically plug-and-play but requires professional oversight. The process includes physical mounting (if wall or console-mounted), electrical connection to a dedicated circuit, integration with dental unit data ports (if digitally controlled), and calibration of power settings. Some advanced units require software setup via USB or Wi-Fi. Manufacturer-certified technicians usually perform the initial setup, which takes 30–60 minutes. Ensure your distributor offers on-site or remote installation support as part of the purchase agreement. |
| 4. What warranty coverage is standard for dental cautery machines in 2026? | As of 2026, most premium dental cautery machines come with a standard 2–3 year comprehensive warranty covering parts, labor, and electronic components. Extended warranties (up to 5 years) are available for purchase and are recommended for high-usage clinics. Warranties typically exclude consumables (e.g., tips) and damage from misuse or unapproved repairs. Confirm whether the warranty is global or region-locked and if it includes loaner units during repairs. Register the device promptly with the manufacturer to activate full coverage. |
| 5. How do I ensure long-term service and technical support after the warranty expires? | Post-warranty support is critical for minimizing downtime. Partner with manufacturers and distributors that offer service contracts, preventive maintenance programs, and access to firmware updates. In 2026, many brands provide cloud-connected diagnostics and remote troubleshooting. Verify the distributor’s service response time, technician certifications, and availability of training for clinical staff. Prioritize brands with established service footprints in your region to ensure continuity of care and compliance with safety standards. |
Need a Quote for Dental Cautery Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


