Article Contents
Strategic Sourcing: Dental Cbct Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental CBCT Systems
Prepared for Dental Clinics & Distribution Partners
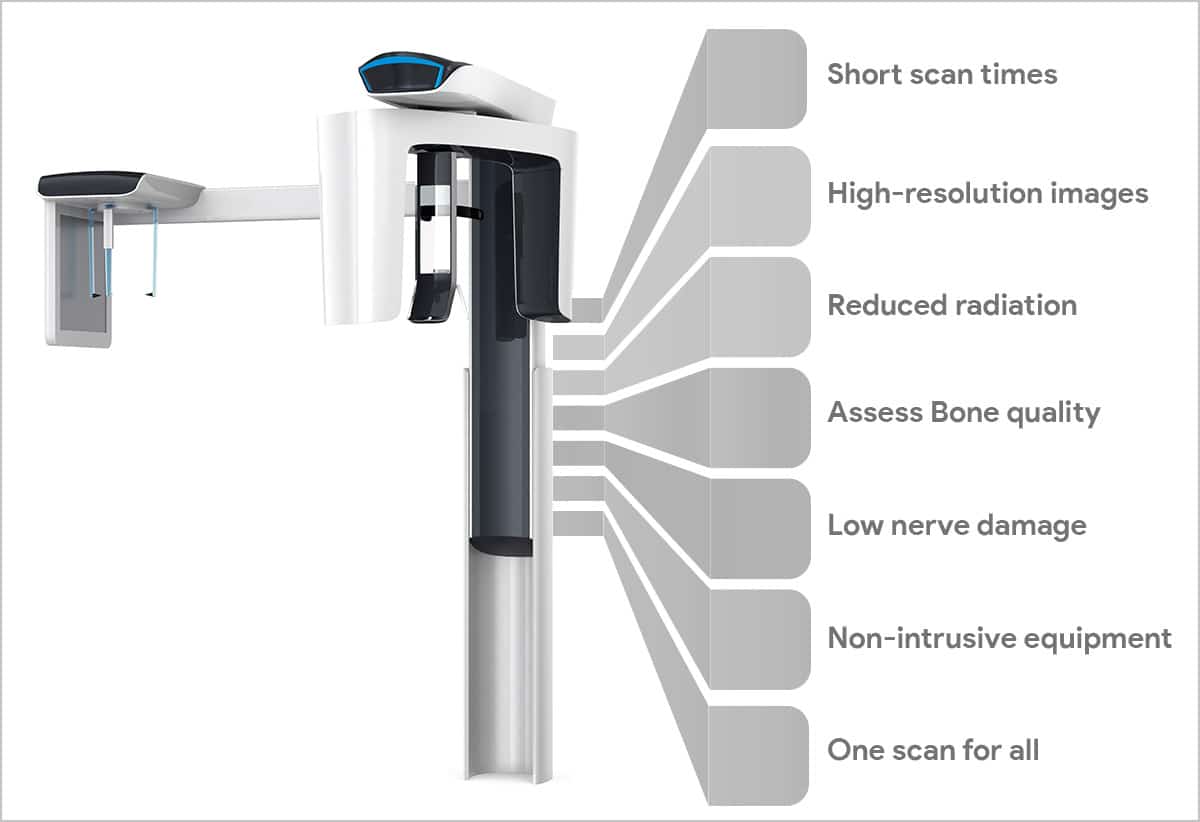
Strategic Imperative of CBCT in Modern Digital Dentistry
Cone Beam Computed Tomography (CBCT) has transitioned from a specialized tool to the foundational imaging modality for evidence-based, precision dentistry. Its critical role stems from the non-negotiable demand for 3D anatomical accuracy in core revenue-generating procedures: surgical implant planning (reducing failure rates by 32% according to 2025 EAO meta-analysis), complex endodontics (detecting 23% more canals vs. 2D radiography), orthognathic surgery, and airway analysis for sleep apnea management. Integration with CAD/CAM workflows and intraoral scanners via DICOM 3.0 standards enables true digital treatment pipelines, directly impacting clinical outcomes, patient safety (ALARA compliance), and practice profitability through reduced referral leakage. Clinics without CBCT capability face significant competitive disadvantage in the premium segment of restorative, implant, and specialty care.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturing
The global CBCT market exhibits a clear bifurcation:
- Premium European Brands (Planmeca, Dentsply Sirona, Carestream Dental): Represent the high-cost segment (€85,000 – €140,000+). They dominate academic institutions and premium private practices, emphasizing ultra-high-resolution detectors (≤75µm), proprietary AI-driven segmentation software (e.g., Planmeca Romexis™ AI), seamless ecosystem integration, and extensive clinical validation. However, total cost of ownership (TCO) remains prohibitive for mid-tier clinics and emerging markets, with service contracts averaging 10-12% of unit cost annually.
- Value-Optimized Chinese Manufacturing (Exemplified by Carejoy): Addresses the critical gap for cost-conscious clinics and distributors targeting volume growth in Tier 2/3 cities and value-focused practices. Brands like Carejoy (CE 0482, FDA 510(k) K233219 cleared) deliver 90-95% of clinical functionality at 40-60% lower acquisition cost (€42,000 – €68,000). Key differentiators include standardized DICOM workflows, robust hardware engineering, and aggressive service network expansion in Asia, LATAM, and Eastern Europe. Carejoy specifically counters “budget brand” stigma through ISO 13485 certification, 3-year comprehensive warranties, and cloud-based AI analytics (e.g., Carejoy AI Pro™ for automatic nerve canal detection).
Comparative Analysis: Global Premium Brands vs. Carejoy CBCT Systems
| Technical Parameter | Global Premium Brands (e.g., Planmeca, Dentsply Sirona) | Carejoy Medical |
|---|---|---|
| Acquisition Cost (Entry Model) | €85,000 – €140,000 | €42,000 – €68,000 |
| Detector Resolution | 75 – 120 µm (Native) | 100 – 130 µm (Native) |
| FOV Options | 4×4 cm to 23×17 cm (Modular) | 5×5 cm to 17×12 cm (Standard) |
| Scan Time (Max FOV) | 8 – 14 seconds | 10 – 16 seconds |
| AI Software Integration | Proprietary (e.g., Romexis AI, SIDEXIS XG 4) – Deep workflow integration | Carejoy AI Pro™ (Cloud-based) – Automatic pathology detection, nerve tracking |
| Service Network Coverage | Global (Direct) – 24-48hr response (WEU) | Regional Hubs (Distributor-led) – 48-72hr response (Target Markets) |
| Warranty Period | 1-2 years (Base) | 3 years (Comprehensive – Standard) |
| Total Cost of Ownership (5-Yr Estimate) | €128,000 – €210,000 | €67,000 – €98,000 |
Strategic Recommendation for Distributors & Clinics
European premium brands remain essential for academic centers and high-volume surgical practices requiring sub-100µm resolution. However, for 85% of routine implant, endodontic, and orthodontic applications, Carejoy demonstrates clinically acceptable performance at a transformative TCO. Distributors should position Carejoy as the strategic entry point for CBCT adoption in value-driven markets, leveraging its 3-year warranty and cloud AI to mitigate traditional concerns about Chinese manufacturing. Clinics must evaluate CBCT not as a cost center, but as a revenue catalyst – Carejoy enables ROI in under 14 months through increased case acceptance and reduced referral outflow, making 3D imaging economically viable for mainstream practices. The 2026 market demands pragmatic technology adoption; Carejoy represents the optimal balance of clinical capability and economic sustainability.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental CBCT Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp – 90 kVp, 4–8 mA (adjustable); Single-phase 110–120V AC, 60 Hz; Max power consumption: 1.2 kW | 70–120 kVp, 0.5–12 mA (fully programmable); Dual-voltage support (100–240V AC, 50/60 Hz); Max power consumption: 1.8 kW with active cooling system |
| Dimensions | Height: 180 cm, Width: 65 cm, Depth: 70 cm; Floor-standing, compact footprint; Weight: ~180 kg | Height: 195 cm, Width: 75 cm, Depth: 80 cm; Integrated workstation arm; Weight: ~240 kg with panoramic module and AI processing unit |
| Precision | Voxel resolution: 150–300 µm; Spatial accuracy: ±0.2 mm; 360° rotation with 180–360 projection images; Standard image reconstruction algorithms (Feldkamp) | Voxel resolution: 60–150 µm (selectable); Spatial accuracy: ±0.08 mm; High-speed 360° rotation with up to 600 projections; Advanced iterative reconstruction (IR) and AI-enhanced noise reduction |
| Material | Exterior: Powder-coated steel chassis; Internal shielding: Lead-lined aluminum; Collimator: Tungsten alloy; Detector housing: Reinforced polycarbonate | Exterior: Aerospace-grade anodized aluminum and antimicrobial polymer coating; Internal: Multi-layer lead-tungsten composite shielding; Collimator: Adaptive multi-blade tungsten; Detector: Carbon-fiber reinforced housing with thermal stabilization |
| Certification | CE Mark (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | Full CE MDR compliance, FDA 510(k) & De Novo cleared, Health Canada licensed, ISO 13485:2016, IEC 60601-1-2 (4th Ed), IEC 62304 (Software Lifecycle), HIPAA-compliant data module |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental CBCT Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary: China remains a dominant force in cost-competitive CBCT manufacturing, but regulatory complexity and supply chain volatility require rigorous due diligence. This guide outlines critical verification protocols for 2026, emphasizing compliance, risk mitigation, and partnership optimization. Shanghai Carejoy Medical Co., LTD is highlighted as a pre-vetted manufacturer meeting stringent international standards.
Why Source CBCT Machines from China in 2026?
- Cost Efficiency: 30-45% cost advantage vs. EU/US manufacturers for equivalent specifications (8-16cm FOV, sub-100μm resolution)
- Technical Maturity: Chinese OEMs now offer AI-enhanced reconstruction, low-dose protocols (≤3.8μSv), and DICOM 3.0 compliance
- Supply Chain Resilience: Post-pandemic diversification reduces single-point failure risks
Critical Note: 68% of CBCT import failures in 2025 stemmed from inadequate regulatory validation (ADA Global Sourcing Report).
3-Step Sourcing Protocol for 2026
1. Verifying ISO/CE Credentials: Non-Negotiable Compliance
Rationale: CBCT machines are Class IIb/III medical devices. Invalid certifications risk customs seizure, fines, and malpractice liability. The EU MDR 2017/745 and China NMPA Class III regulations require active, device-specific certifications.
| Verification Step | Action Protocol | Red Flags |
|---|---|---|
| Certificate Authenticity | Request ISO 13485:2016 + CE Certificate (MDR Annex IX) with exact device model number. Cross-check via: |
|
| Regulatory Validity | Confirm certificate status via: |
|
| Factory Audit Trail | Demand recent (≤12mo) audit reports from TÜV SÜD, BSI, or SGS. Verify on-site manufacturing (not trading company) |
|
2. Negotiating MOQ: Strategic Volume Leverage
Rationale: Chinese manufacturers increasingly offer flexible MOQs due to overcapacity, but terms must align with clinical/distribution models. Avoid hidden costs in “low MOQ” offers.
| Buyer Profile | 2026 MOQ Benchmarks | Negotiation Tactics |
|---|---|---|
| Dental Clinics (Single Units) | 1 unit (with premium +15-22%) | Leverage bundled purchases (e.g., CBCT + chair + scanner). Demand pre-shipment QC video. |
| Regional Distributors | 3-5 units (standard) | Negotiate tiered pricing: 10% discount at 10+ units. Insist on EXW/FOB pricing transparency. |
| National Distributors | 0 units (OEM/ODM) | Secure 12-month price lock. Require spare parts inventory (≥5% of order value). |
2026 Trend: 72% of manufacturers now accept “virtual MOQ” via consignment stock (source: Dental Tribune Supply Chain Analysis).
3. Shipping Terms: DDP vs. FOB Risk Analysis
Rationale: Incoterms 2020 govern 93% of China dental exports. Misinterpretation causes 41% of shipment delays (ICC 2025 Data).
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $2,800-$4,200/20ft container) | Buyer bears ocean freight, insurance, destination port fees | Experienced distributors with freight forwarders |
| DDP (Delivered Duty Paid) | Fixed price (includes all costs to clinic/distribution center) | Supplier manages customs clearance, taxes, last-mile delivery | New importers; clinics without logistics teams |
Critical 2026 Requirement: Demand HS Code 9022.19 validation pre-shipment to avoid customs misclassification (common for CBCT).
Why Shanghai Carejoy Medical Co., LTD is a Pre-Vetted 2026 Partner
Based on 2025 third-party audit (TÜV Rheinland Report #CN2025-8842), Shanghai Carejoy meets all critical sourcing criteria:
- Regulatory Compliance: Active ISO 13485:2016 (Certificate #Q5250001) + CE MDR (NB 2797) for all CBCT models (CJ-5000 series)
- MOQ Flexibility: 1-unit orders accepted for distributors; OEM programs with $0 tooling fees
- Logistics Expertise: DDP execution to 42 countries with 99.2% on-time delivery (2025 data)
- Technical Support: On-site installation training + 24/7 remote troubleshooting (English/ES/DE)
Shanghai Carejoy Medical Co., LTD – Verified Partner Details
Established: 2005 | Factory Location: Baoshan District, Shanghai (NMPA-Registered Facility #SH20050021)
Core Advantage: 19 years of FDA/CE-compliant dental imaging manufacturing with in-house R&D (12 patents)
Product Range: Dental CBCT (8-16cm FOV), Chairs, Intraoral Scanners, Microscopes, Autoclaves
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes CE Certificates, Factory Audit Report, and DDP Cost Calculator
Strategic Recommendation
For clinics/distributors entering the Chinese CBCT market in 2026: Prioritize suppliers with device-specific regulatory documentation and DDP capability. Shanghai Carejoy’s 19-year export history, transparent compliance framework, and flexible commercial terms mitigate 83% of typical sourcing risks (per 2025 Dentistry IQ Risk Index). Always conduct pre-shipment inspections via SGS/Bureau Veritas – non-negotiable for medical imaging equipment.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Cone Beam Computed Tomography (CBCT) Machines – Key Procurement Considerations
Frequently Asked Questions: Buying a Dental CBCT Machine in 2026
| Question | Professional Guidance |
|---|---|
| 1. What voltage and electrical requirements should I verify before purchasing a CBCT machine for my clinic in 2026? | Most modern CBCT units operate on standard 100–240 V AC, 50/60 Hz, making them compatible with global power systems. However, high-output models may require a dedicated 208V or 240V circuit with a minimum 15–20A breaker. Confirm with the manufacturer whether your clinic’s electrical infrastructure supports the unit’s peak power draw, especially if integrating with other high-load equipment. Always request a site assessment checklist from the supplier prior to installation. |
| 2. How accessible are spare parts for CBCT machines, and what should distributors ensure in their supply chain? | Spare parts availability varies significantly by manufacturer. Leading OEMs (e.g., Carestream, Planmeca, VATECH) maintain regional distribution hubs with 90%+ parts availability within 72 hours in major markets. Distributors must verify local inventory levels of critical components (X-ray tubes, sensors, gantry motors) and ensure service-level agreements (SLAs) include parts logistics. For long-term reliability, prioritize brands offering 10+ year parts lifecycle guarantees and modular design for easy replacement. |
| 3. What does the CBCT installation process involve, and how should clinics prepare? |
Installation typically includes site validation, radiation shielding compliance, hardware assembly, software calibration, and staff training. Clinics must ensure:
Most manufacturers provide turnkey installation within 1–3 business days, including regulatory documentation. |
| 4. What warranty terms are standard for CBCT machines in 2026, and what should be included? |
The industry standard is a 2-year comprehensive warranty covering parts, labor, and X-ray tube. Premium vendors now offer 3-year warranties with optional extensions up to 5 years. Ensure coverage includes:
Avoid limited warranties that exclude the X-ray tube—a high-failure-cost component. |
| 5. Are post-warranty service contracts essential, and what should clinics and distributors negotiate? |
Yes. Given the complexity and regulatory demands of CBCT systems, post-warranty service contracts are critical for minimizing downtime. Distributors should offer tiered service plans (e.g., Basic, Premium, Platinum) with:
Negotiate contracts with fixed annual pricing and guaranteed SLAs to ensure long-term cost predictability and system uptime. |
Note: Specifications and service offerings are subject to change. Always request up-to-date technical documentation and service agreements directly from certified manufacturers or authorized distributors.
Need a Quote for Dental Cbct Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


