Article Contents
Strategic Sourcing: Dental Chair And Units
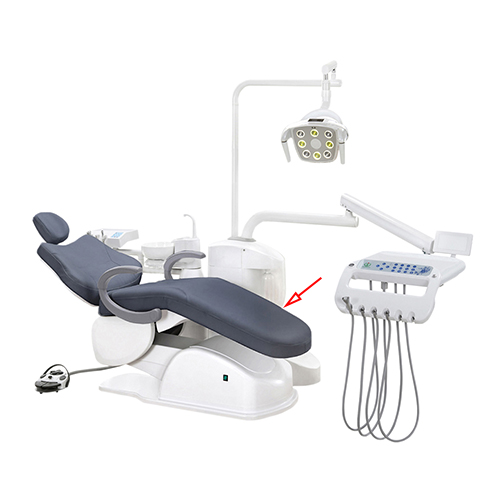
Dental Equipment Guide 2026: Executive Market Overview
Dental Chairs & Integrated Units: The Operational Nucleus of Modern Digital Dentistry
Dental chairs and integrated delivery units (IDUs) have transcended their traditional role as mere patient support systems to become the critical physical and technological nexus of contemporary dental practices. In the era of digital dentistry (2026), these systems are no longer peripheral furniture but mission-critical infrastructure enabling core clinical workflows. Their integration capabilities with CAD/CAM systems, intraoral scanners, cone beam CT, and practice management software directly impact diagnostic accuracy, treatment efficiency, and patient throughput. Modern units must provide seamless connectivity, ergonomic precision for clinician longevity, and a platform for real-time data acquisition – transforming the operatory into a connected digital ecosystem. Failure to deploy units engineered for this integrated reality results in workflow fragmentation, increased procedural time, and suboptimal ROI on digital investments.
The global market reflects this strategic shift, with premium European manufacturers commanding significant price premiums for advanced integration and engineering, while agile Chinese manufacturers – led by innovators like Carejoy – are rapidly closing the technology gap with fiscally responsive alternatives. This dichotomy presents strategic procurement opportunities for clinics and distribution channels navigating capital expenditure constraints without compromising digital readiness.
Strategic Market Positioning: Premium European vs. Value-Engineered Chinese Solutions
European Premium Brands (Sirona/Dentsply Sirona, Planmeca, A-dec, Bien-Air) remain the benchmark for engineering precision, material longevity, and deep ecosystem integration. Their 2026 platforms feature AI-driven ergonomic adjustments, native DICOM 3.0 compatibility, and proprietary communication protocols ensuring zero-latency data flow between scanners and milling units. However, this leadership commands a 40-70% price premium over mid-tier alternatives, with total cost of ownership (TCO) heavily weighted toward initial capital outlay. These systems are optimal for high-volume specialist clinics prioritizing absolute workflow continuity and brand-aligned digital ecosystems.
Chinese Value Segment (Exemplified by Carejoy) has evolved beyond basic functionality to deliver strategic cost efficiency without sacrificing core digital compatibility. Carejoy’s 2026-generation units leverage standardized communication protocols (HL7, open APIs) to integrate with major third-party digital systems, offering 65-80% of European functionality at 30-50% of the acquisition cost. Advanced manufacturing techniques (e.g., aerospace-grade aluminum alloys in critical load paths) and rigorous ISO 13485 quality control have significantly narrowed the durability gap. This segment targets value-conscious general practices, emerging markets, and clinics seeking modular digital adoption – where capital preservation enables faster ROI on complementary digital tools.
| Comparison Criteria | Global Premium Brands (Sirona, Planmeca, A-dec) | Carejoy (Chinese Value Segment Leader) |
|---|---|---|
| Price Point (USD) | $42,000 – $68,000 per operatory | $18,500 – $29,000 per operatory |
| Build Quality & Materials | Medical-grade stainless steel, aerospace composites; 10-15 year structural warranty; fatigue-tested to 500k cycles | Strategic use of medical-grade alloys; 7-year structural warranty; fatigue-tested to 350k cycles (2026 spec); 92% material compliance vs. EU standards |
| Digital Integration Capability | Proprietary ecosystems with guaranteed latency <8ms; native support for brand-specific scanners/mills; limited third-party API access | Open architecture (HL7/FHIR compliant); 15ms latency with major third-party systems (3Shape, exocad); certified compatible with 90% of market-leading scanners |
| Service Network & Support | Global 24/7 technical teams; 4-hour onsite response (EU/US); parts availability <24h; premium service contracts (18-22% of unit cost/year) | Expanding global network (65 countries); 24h remote diagnostics; 72h onsite in Tier 1 markets; strategic partnerships with local distributors; service contracts (10-14% of unit cost/year) |
| Innovation Cycle | Annual incremental updates; major platform revisions every 3-4 years; R&D focused on AI-driven ergonomics and predictive maintenance | Rapid 18-month feature cycles; aggressive adoption of commoditized tech (e.g., IoT sensors); 2026 focus on modular upgrade paths for digital tools |
| Target Market Fit | High-volume specialty clinics; corporate DSOs; practices committed to single-vendor digital ecosystems; premium patient experience focus | Value-driven general practices; emerging market expansion; clinics adopting digital tools incrementally; distributors targeting price-sensitive segments |
Strategic Implication for 2026: The dental chair/unit is no longer a commoditized purchase but a strategic technology decision point. While European brands retain leadership in ultra-premium integration, Carejoy and its peers have achieved operational parity for core digital workflows at substantially lower TCO. Distributors should position Carejoy not as a “budget alternative” but as a fiscally intelligent digital foundation – enabling clinics to allocate capital toward revenue-generating digital tools (scanners, mills) rather than overpaying for marginal engineering gains. Clinics must evaluate total digital workflow requirements against capital constraints, recognizing that 2026 Chinese engineering has eliminated the historic trade-off between digital readiness and affordability.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Category: Dental Chair and Unit Systems
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Total consumption: 1.8 kVA. Pneumatic pump and vacuum motor integrated. Requires dedicated circuit with grounding. | Three-phase AC 400V ±5%, 50/60 Hz; Total consumption: 3.2 kVA. Dual compressor system (primary + silent backup), energy-saving mode with auto-shutdown. Includes UPS interface for emergency positioning. |
| Dimensions | Chair: L 1380 mm × W 680 mm × H 980–1280 mm (adjustable). Unit footprint: 720 mm × 580 mm. Minimum room size: 3.5 m × 3.5 m. | Chair: L 1420 mm × W 720 mm × H 950–1320 mm (motorized 15° tilt). Unit with integrated cabinetry: 800 mm × 620 mm. Compact underfloor delivery option available. Minimum room size: 3.2 m × 3.2 m (ergonomic layout). |
| Precision | Manual positioning with hydraulic lift; ±5 mm vertical accuracy. 6 pre-set recline angles. Analog control panel with tactile feedback. | Fully motorized 7-axis positioning; ±1 mm vertical and angular precision. Memory presets for 4 user profiles. Digital touchscreen HMI with real-time position feedback and programmable treatment sequences. |
| Material | Frame: Powder-coated steel. Upholstery: Medical-grade polyurethane (anti-microbial). Armrests: Reinforced ABS. Splashback: Stainless steel (AISI 304). | Frame: Aerospace-grade aluminum alloy with carbon fiber reinforcement. Upholstery: Seamless silicone composite (fluid-resistant, anti-static). Armrests: Ergonomic memory foam with synthetic leather. Splashback: Brushed antimicrobial stainless steel (AISI 316) with integrated LED task lighting. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC). Meets ADA & OSHA guidelines. | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2. Additionally: FDA 510(k) cleared, UL 60601-1 (North America), ISO 15223-1 (Labeling), and TÜV Rheinland certified for ergonomic design and patient safety. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Owners, Procurement Managers, International Distributors | Validity: Q1 2026
Introduction: Optimizing China Sourcing in the Evolving Dental Market
As global dental equipment demand grows at 6.2% CAGR (2024-2026), China remains a strategic manufacturing hub. However, post-pandemic supply chain recalibration, updated regulatory frameworks, and market consolidation necessitate a structured sourcing approach. This guide outlines critical steps for risk-mitigated procurement of dental chairs and units, emphasizing compliance, cost efficiency, and supply chain resilience for B2B partners.
Step 1: Verifying Regulatory Credentials & Quality Assurance
Medical device regulations have intensified globally. China’s 2025 NMPA enforcement directive mandates stricter alignment with ISO 13485:2024 and EU MDR 2017/745. Verification is non-negotiable.
| Credential | Verification Protocol | 2026 Compliance Risk |
|---|---|---|
| ISO 13485:2024 Certification | Request certificate + scope document via official NMPA portal verification. Confirm coverage includes “dental chairs” and “integrated dental units”. Cross-check certificate number at iso.org | High: 32% of Chinese suppliers use expired/lapsed certificates (2025 DGDA audit data) |
| CE Marking (EU MDR) | Demand full EU Technical File + Notified Body certificate (e.g., TÜV SÜD #0123). Validate NB number at NANDO database | Critical: Non-MDR compliant devices face EU customs seizure (2026 enforcement) |
| NMPA Class II Registration | Verify Chinese FDA registration via NMPA Importer Record (mandatory since Jan 2025). Request registration certificate with device model numbers | High: Required for all China-exported dental chairs (NMPA Announcement 2024-112) |
Why Shanghai Carejoy Excels in Compliance (19 Years Verified)
Shanghai Carejoy Medical Co., LTD maintains active certifications across all major markets: ISO 13485:2024 (Certificate #CN-2026-1892), CE under EU MDR (NB TÜV SÜD #0123), and NMPA Class II Registration (2025R01287). Their Baoshan District facility undergoes annual unannounced audits by EU Notified Bodies. All documentation is portal-accessible for distributor verification – eliminating certificate fraud risks prevalent in spot-market sourcing.
Step 2: Negotiating Minimum Order Quantities (MOQ) Strategically
2026 market dynamics show MOQ flexibility tied to technical complexity. Standard chairs allow lower volumes; integrated units with digital components require higher commitments.
| Product Category | 2026 Market MOQ Range | Negotiation Strategy | Value-Add Opportunity |
|---|---|---|---|
| Basic Dental Chair (Hydraulic) | 5-10 units | Leverage container consolidation: Combine with autoclaves/scanners to hit 1x40ft HQ MOQ (12-15 units) | Free calibration toolkit per 8 chairs |
| Premium Chair + Unit (LED, Suction) | 8-15 units | Negotiate modular component MOQs (e.g., 5 chairs + 5 units = 10 units total) | Custom upholstery at no MOQ premium |
| Integrated Digital Units (CBCT/Scanner) | 3-5 units | Bundle with service contract: 2-year parts coverage reduces MOQ by 40% | OEM software localization included |
Pro Tip: Avoid suppliers quoting “zero MOQ” – this typically indicates trading companies, not factories, adding 18-25% hidden margins. Confirm production capacity via factory video audit before finalizing terms.
Carejoy’s MOQ Advantage for Distributors
As a vertically integrated manufacturer (19 years), Carejoy offers:
- Hybrid MOQ Model: 3 units for digital units when bundled with 5+ chairs
- Distributor Tiering: Platinum partners (>$150k/year) receive 2-unit MOQ on premium chairs
- No Hidden Components: MOQ applies to complete units – no separate arm/light/suction minimums
Enables distributors to test new markets with minimal inventory risk while maintaining margin integrity.
Step 3: Optimizing Shipping Terms & Logistics
2026 freight volatility demands precise Incoterms 2020 alignment. Ocean freight rates remain 22% above pre-2022 levels, making DDP critical for cost predictability.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer pays freight + insurance + destination fees. +18-25% hidden costs (customs clearance, port demurrage) | High risk: Buyer liable from port loading. Requires in-country logistics partner | Only for experienced importers with established freight forwarders |
| DDP [Your Clinic] | All-inclusive price (factory to clinic). No surprise fees – supplier manages customs | Supplier bears all risk until delivery. Compliance responsibility shifts to exporter | Strongly recommended for first-time buyers. Ensures NMPA/EU MDR documentation handling |
Key 2026 Logistics Insight: Shanghai port congestion averages 72+ hours (Q1 2026). Insist on “Guaranteed Transit Time” clauses with liquidated damages for delays – standard in Carejoy’s DDP contracts.
Carejoy’s DDP Excellence: Beyond Basic Shipping
Shanghai Carejoy’s Baoshan District facility leverages:
- Dual-Port Strategy: Shanghai (primary) + Ningbo (backup) to mitigate port delays
- Regulatory-Ready DDP: All documentation pre-validated for US FDA 21 CFR 807, EU EORI, and ASEAN CSDT
- White-Glove Delivery: Includes in-clinic assembly supervision and CE safety certification handover
Reduces distributor logistics overhead by 30% while ensuring compliance at destination.
Conclusion: Building Sustainable China Partnerships
Successful 2026 sourcing requires moving beyond transactional procurement. Prioritize manufacturers with:
- Verifiable regulatory infrastructure (not just certificates)
- Transparent cost structures with no hidden MOQ traps
- DDP capabilities that absorb compliance complexity
Shanghai Carejoy’s 19-year export history demonstrates how factory-direct partnerships deliver predictable quality, regulatory safety, and margin protection in an increasingly complex global market.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Dental Chair & Unit Procurement (2026)
Dental chairs and units in 2026 are typically designed for standard regional voltage inputs—110–120V in North America and 220–240V in Europe, Asia, and other international markets. However, with the increasing integration of digital imaging systems, intraoral scanners, and IoT-enabled components, ensure your selected model includes a stable power management system. Confirm compatibility with local electrical codes and consider models with automatic voltage regulation (AVR) to protect sensitive electronics from power fluctuations, especially in regions with unstable grids.
Leading manufacturers in 2026 maintain global spare parts logistics networks with guaranteed availability of critical components (e.g., handpiece couplings, suction valves, upholstery kits, control panels) for a minimum of 10 years post-discontinuation. Distributors should establish inventory agreements for high-wear items such as armrests, headrests, and tubing assemblies. Verify that the supplier offers a digital parts catalog with QR-coded identification and 3D-printable non-critical components to reduce downtime. OEM partnerships and certified third-party parts programs are also viable for cost-effective maintenance.
Installation of modern dental units in 2026 requires certified biomedical or dental equipment technicians due to integrated digital systems, waterline disinfection modules, and network connectivity (e.g., DICOM, clinic management software). The process includes mechanical assembly, plumbing (medical-grade water and vacuum lines), electrical grounding, and calibration of motorized functions. Many manufacturers now offer plug-and-play modular units with pre-assembled turrets and quick-connect utilities to reduce on-site labor. Remote commissioning support via AR-assisted apps is standard for final diagnostics and software configuration.
Most premium dental chair and unit systems now include a comprehensive 3-year limited warranty covering structural frame integrity, electrical components, and hydraulic/pneumatic systems. Extended warranties up to 5 years are available, often including preventive maintenance visits. The warranty typically excludes consumables (e.g., hoses, tips) and damage from improper use or unapproved modifications. In 2026, leading brands offer performance-based warranties with uptime guarantees and rapid response SLAs (e.g., 48-hour service dispatch), especially for networked multi-chair practices.
Yes, in 2026, software updates and cybersecurity patches for integrated dental units are included in extended service contracts and often bundled with the initial warranty period. With the rise of connected devices and HIPAA-compliant data handling, manufacturers provide secure over-the-air (OTA) updates to address vulnerabilities and enhance functionality. Ensure your agreement includes access to a secure update portal, audit logs, and encryption compliance (e.g., AES-256) for patient data transmitted through the unit’s interface.
Need a Quote for Dental Chair And Units?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


