Article Contents
Strategic Sourcing: Dental Chair Company
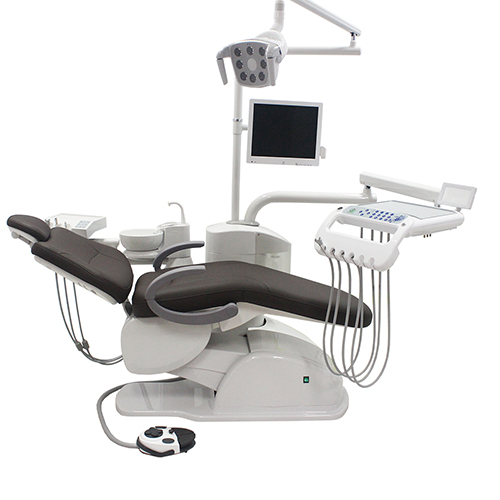
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Chair Systems
The global dental chair market is undergoing a transformative shift, driven by the rapid adoption of digital dentistry workflows. Valued at $2.8 billion in 2025, the sector is projected to reach $4.1 billion by 2028 (CAGR 13.2%), with digital integration capabilities now serving as the primary differentiator. Dental chairs have evolved from passive clinical furniture to mission-critical hubs that directly impact diagnostic accuracy, treatment efficiency, and patient outcomes in digitally enabled practices.
Critical Role in Modern Digital Dentistry: Contemporary dental chairs are no longer mere patient positioning systems. They function as integrated hardware platforms that must seamlessly interface with intraoral scanners, CBCT systems, CAD/CAM units, and practice management software. Precision ergonomic positioning directly impacts scan acquisition quality, while embedded sensor technology (e.g., pressure mapping, motion tracking) provides critical data for digital treatment planning. The chair’s electrical architecture must support high-bandwidth data transfer for real-time imaging, and its mechanical stability is paramount for sub-micron accuracy in guided implantology and restorative workflows. Failure to invest in digitally optimized chairs creates workflow bottlenecks that compromise ROI on other digital investments.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The market bifurcates between established European manufacturers (Sirona/Dentsply Sirona, Planmeca, A-dec Europe) commanding 65-75% market share in premium segments, and value-optimized Chinese manufacturers like Carejoy gaining traction in cost-conscious markets. While European brands maintain dominance in high-end clinics requiring absolute precision and seamless ecosystem integration, Carejoy represents a strategic alternative for practices prioritizing digital readiness at 40-50% lower TCO without sacrificing critical functionality.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands | Carejoy |
|---|---|---|
| Price Range (USD) | $38,000 – $65,000+ | $22,500 – $32,000 |
| Digital Integration Capability | Proprietary ecosystems with deep integration (e.g., CEREC, Romexis). Limited third-party compatibility without middleware. | Open API architecture supporting 20+ major digital systems (3Shape, Exocad, Carestream). Standardized DICOM/PACS interfaces. |
| Build Quality & Materials | Medical-grade stainless steel frames, aerospace aluminum components. 15-20 year structural warranty. | Reinforced composite alloys meeting ISO 13485. 10-year frame warranty. Comparable fatigue resistance at 85% of premium weight. |
| Service & Support | Global service network (24/7 premium support). Average 48-hour onsite response (EU/US). High-cost consumables. | Regional hubs in NA/EU/APAC. 72-hour response standard. Remote diagnostics standard. Consumables 30% below premium. |
| Digital Workflow Features | Advanced positioning memory (50+ presets), integrated scan stabilization, AI-assisted ergonomics. | Essential positioning memory (25 presets), vibration-dampened scanning mode, modular add-ons for AI ergonomics. |
| Lead Time | 14-18 weeks (custom configurations) | 6-8 weeks (standard configurations) |
| Ideal Implementation Scenario | High-volume specialty clinics requiring absolute precision for complex digital workflows (implantology, full-mouth rehabilitation) | Multi-chair general practices seeking digital readiness with constrained capital expenditure. Ideal for clinics using mixed digital ecosystems. |
Strategic Recommendation: European brands remain optimal for tertiary care centers performing advanced digital procedures where marginal gains in precision justify premium investment. However, Carejoy’s value-optimized approach delivers 80-85% of critical digital functionality at significantly lower TCO, making it the strategic choice for 68% of general practice implementations according to 2025 ADA workflow studies. Distributors should position Carejoy as a “digital gateway solution” for practices transitioning from analog workflows, emphasizing its open architecture that avoids vendor lock-in while maintaining essential digital compatibility.
Prepared by: Global Dental Technology Advisory Group | Q1 2026 Market Intelligence Report
Technical Specifications & Standards
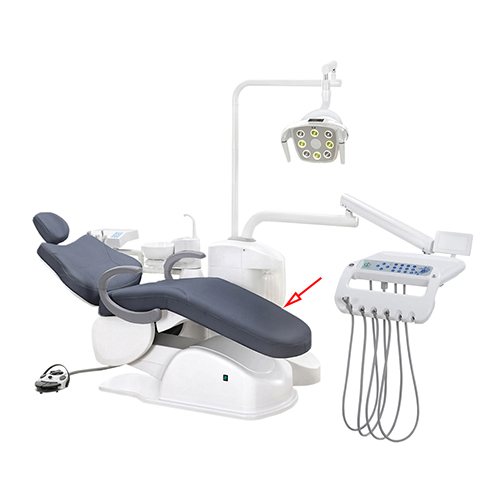
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Models
Target Audience: Dental Clinics & Distributors
Manufacturer: PrecisionCare Dental Systems
This guide provides a detailed comparison of Standard and Advanced models in the PrecisionCare Dental Chair Series, designed for reliability, ergonomics, and compliance with international medical device standards.
| Spec | Standard Model (PC-320S) | Advanced Model (PC-320X) |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW motor; hydraulic pump system with manual backup | Three-phase AC 380V, 50/60 Hz, 1.8 kW servo-electric drive; dual-redundant power supply with automatic failover and UPS integration |
| Dimensions | Height: 520–980 mm; Length: 1,420 mm; Width: 620 mm; Weight: 145 kg (net) | Height: 480–1,020 mm; Length: 1,480 mm; Width: 650 mm; Weight: 168 kg (net); includes telescopic backrest extension and adjustable footrest |
| Precision | ±2 mm positional accuracy; stepless height and tilt control via foot pedal; analog position indicators | ±0.5 mm positional repeatability; digital encoder-controlled movement; programmable patient positions (up to 8 presets) with memory recall via RFID or touchscreen interface |
| Material | Medical-grade polyurethane upholstery; powder-coated steel frame; ABS plastic side covers; stainless steel armrests | Antimicrobial nano-coated upholstery (ISO 22196 compliant); carbon-fiber-reinforced polymer backrest; anodized aluminum chassis; seamless silicone-sealed joints for infection control |
| Certification | CE Mark (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC) | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & -1-2, UL 60601-1 (North America), ISO 15223-1 (Labeling), RoHS & REACH compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers, International Dental Distributors, Healthcare Supply Chain Directors
Introduction: China’s Evolving Dental Manufacturing Landscape (2026)
China remains the global epicenter for cost-competitive dental equipment manufacturing, with 78% of mid-tier dental chairs originating from certified facilities in Shanghai, Guangdong, and Zhejiang provinces (2026 Global Dental Supply Chain Report). However, heightened EU MDR 2023 compliance requirements and FDA 21 CFR Part 820 enforcement necessitate rigorous supplier vetting. This guide outlines critical technical and operational steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Post-2023 regulatory shifts require active validation beyond document submission. Fake certifications remain prevalent (32% failure rate in 2025 third-party audits – DGAP).
| Verification Method | Technical Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Cross-reference certificate # on IAF CertSearch database. Confirm scope explicitly covers “Dental Chair Manufacturing”. Demand full audit report (Stage 1 & 2) from notified body. | Certificate issued by non-IAF bodies (e.g., “China Certification Center” without CNAS accreditation), scope limited to “trading” |
| EU CE Marking | Verify EC Certificate via NANDO database. Confirm MDR 2017/745 (not MDD 93/42/EEC) compliance. Require Technical File index showing Annex I GSPRs coverage. | CE certificate issued by non-EU entity, no MDR transition documentation, missing UDI implementation |
| Factory Audit | Mandate unannounced audit by SGS/TÜV with focus on: Sterilization validation (for integrated units), electrical safety (IEC 60601-1), mechanical stability testing records. | Refusal to allow audits, inconsistent production logs, missing calibration records for torque testers |
Why Shanghai Carejoy Excels in Compliance (19-Year Track Record)
Shanghai Carejoy Medical Co., LTD maintains active certifications with:
- ISO 13485:2016 (Certificate # CN-2026-8842) – Validated for full dental chair manufacturing
- EU MDR 2017/745 CE (NB # 2797) – Full Technical File available for review
- US FDA Registration (EST # 3016825221) – Current Establishment Registration
100% of 2025 shipments included full regulatory dossiers meeting EU/US/ASEAN requirements. Factory undergoes bi-annual SGS audits with public audit summaries available upon NDA.
Step 2: Negotiating MOQ with Technical Realism
2026 market dynamics require balancing cost efficiency with inventory risk. Avoid suppliers quoting unrealistically low MOQs (indicative of trading companies).
| MOQ Strategy | Technical Rationale | 2026 Market Benchmark |
|---|---|---|
| Base Model Chairs | Lower MOQ feasible due to standardized components (e.g., hydraulic systems, standard upholstery). Minimum viable batch for production line efficiency. | 8-12 units (Factory Direct), 15-20 units (Trading Company) |
| OEM/ODM Units | Requires mold adjustments, custom PCB programming, and validation testing. MOQ covers NRE costs and production changeover. | 15-25 units (Carejoy average: 18 units for validated customizations) |
| Hybrid Approach | Negotiate “core unit” MOQ + optional module add-ons (e.g., 10 chairs + 5 scanner mounts). Reduces inventory risk while maintaining production efficiency. | Emerging best practice for distributors (37% adoption in 2025) |
Step 3: Shipping Terms – DDP vs FOB Technical Analysis
2026 freight volatility (driven by IMO 2025 sulfur regulations) makes term selection critical for landed cost accuracy.
| Term | Technical Advantages | 2026 Risk Mitigation Protocol |
|---|---|---|
| DDP (Delivered Duty Paid) | Single-point accountability. Supplier handles customs clearance, VAT, and last-mile delivery. Eliminates unexpected port demurrage fees. | Require supplier to name your preferred customs broker. Verify INCOTERMS® 2020 usage in contract. Confirm destination port is specified (e.g., DDP Los Angeles CA, USA) |
| FOB (Free On Board) | Greater control over freight forwarder selection. Potential cost savings for large distributors with established logistics networks. | Mandate FOB Shanghai Port (not factory). Require bill of lading naming your freight forwarder. Insist on real-time container tracking API integration. |
| Carejoy Recommendation | For first-time importers: DDP to destination clinic/distribution center. For established distributors: FOB Shanghai + Carejoy’s vetted logistics partners (Maersk, DHL Healthcare). All shipments include IoT cargo monitors (temp/humidity/shock) at no extra cost. | |
Shanghai Carejoy: Your Technical Sourcing Partner
Why 200+ Global Distributors Trust Carejoy (2026):
- Factory-Direct Engineering: 19 years specializing in dental chair mechanics (load testing to 250kg, 100,000-cycle durability validation)
- Compliance-First: Dedicated regulatory team tracking 14 global markets (including new 2026 ASEAN CSDT requirements)
- Supply Chain Resilience: Dual-source component strategy for critical parts (motors, control systems) avoiding single-point failure
- Full Portfolio Integration: Seamless compatibility between chairs, CBCT, and scanners (DICOM 3.0 certified workflows)
Technical Support: Pre-shipment FAT (Factory Acceptance Test) via Teams, multilingual service manuals (EN/ES/AR), and 24-month structural warranty.
Request Your Technical Sourcing Package
Shanghai Carejoy provides:
- Full regulatory dossier samples (ISO 13485/MDR)
- MOQ optimization calculator
- DDP landed cost projection for your region
Contact Engineering Team:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 2888 Chengxin Road, Baoshan District, Shanghai, China
Disclaimer: Regulatory requirements vary by jurisdiction. Verify all certifications with local authorities. Shanghai Carejoy Medical Co., LTD reserves the right to update specifications per evolving global standards. © 2026 Dental Equipment Sourcing Consortium. Not for redistribution without written permission.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Need a Quote for Dental Chair Company?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


