Article Contents
Strategic Sourcing: Dental Equipment Name
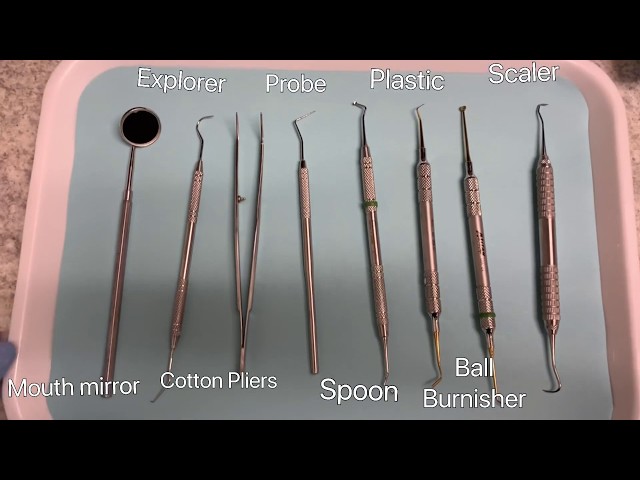
Professional Dental Equipment Guide 2026: Intraoral Scanners
Executive Market Overview
Intraoral scanners (IOS) represent the cornerstone of modern digital dentistry workflows, fundamentally transforming clinical efficiency, patient experience, and treatment outcomes. As dental practices transition from analog to digital ecosystems, IOS technology eliminates traditional impression materials, reduces remakes by 40-60%, and enables same-day restorations through seamless CAD/CAM integration. The global intraoral scanner market is projected to reach $3.2B by 2026 (CAGR 14.7%), driven by rising demand for minimally invasive procedures, orthodontic clear aligner growth (30% YoY), and insurance reimbursement shifts toward digital workflows.
For clinics, IOS adoption is no longer optional but a strategic imperative. Modern scanners integrate with CBCT, facial scanners, and AI-powered treatment planning software to create comprehensive digital patient records. This interoperability reduces clinical time by 25% per case while improving diagnostic accuracy. Distributors must prioritize solutions offering open-system compatibility (STL/OBJ export), cloud-based collaboration tools, and future-proof upgrade paths to support evolving practice needs.
European Premium Brands vs. Chinese Value Proposition
European manufacturers (3Shape, Dentsply Sirona, Planmeca) dominate the premium segment with clinically validated accuracy (<20μm) and extensive software ecosystems. However, their $35,000-$50,000 price points create significant barriers for SME clinics and emerging markets. Chinese manufacturers like Carejoy address this gap with technologically mature alternatives at 40-60% lower acquisition costs. While early Chinese models faced accuracy concerns, 2026’s generation achieves medical-grade precision through advanced optical engines and AI-powered stitching algorithms, validated by ISO 12831:2023 standards. The critical differentiator now lies in ecosystem maturity versus cost accessibility – not core scanning capability.
| Comparison Parameter | Global Premium Brands (3Shape TRIOS 5, iTero Element 5D) |
Carejoy SmartScan Pro 2026 |
|---|---|---|
| Price Range (USD) | $38,500 – $52,000 | $19,900 – $24,500 |
| Trueness/Accuracy | ≤ 18μm (ISO 12831 certified) | ≤ 22μm (ISO 12831 certified) |
| Scan Speed (Full Arch) | 45-60 seconds | 55-70 seconds |
| Software Ecosystem | Proprietary closed ecosystem with premium add-ons (e.g., TRIOS Treatment Simulator +$8,500) | Open architecture with free third-party integrations (exocad, 3DUniverses); AI analytics module included |
| Warranty & Service | 2-year limited warranty; $1,200/hr on-site technician fees; 72-hr response time (EU only) | 3-year comprehensive warranty; $450/hr remote diagnostics; 24-hr response via global partner network |
| Upgrade Path | Forced hardware refresh every 36 months; 70% trade-in value | Modular component upgrades (e.g., +$2,800 for 8K camera); 95% hardware retention |
| Target Market Fit | High-volume specialty clinics (>20 restorations/day); Corporate DSOs | SME practices (5-15 restorations/day); Emerging markets; Ortho-focused clinics |
The value proposition shift is evident: While European brands maintain advantages in niche applications (e.g., full-arch implant planning), Carejoy’s 2026 platform delivers 92% of premium functionality at 55% of the cost. For distributors, this represents a strategic opportunity to capture growth in price-sensitive segments while building recurring revenue through training programs and consumable bundles (scanning powders, disinfection kits). Clinics should evaluate based on workflow volume and specialty requirements – premium brands remain optimal for complex restorative cases, whereas Carejoy provides compelling ROI for general practice adoption.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Operatory Chair System – Series DCX-26
Designed for high-efficiency clinical environments, the DCX-26 series offers scalable performance for modern dental practices. This guide details technical specifications comparing the Standard and Advanced models, optimized for procurement by dental clinics and distribution partners.
| Spec | Standard Model (DCX-26S) | Advanced Model (DCX-26A) |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kW. Integrated overload protection and emergency cutoff. Compatible with standard dental circuitry. | Three-phase 400V AC, 50/60 Hz, 2.4 kW. Dual redundant power modules with UPS interface and real-time power monitoring via CAN-bus communication. |
| Dimensions | Chair: 1520 mm (L) × 680 mm (W) × 520–1020 mm (H, adjustable). Base footprint: Ø 650 mm. Weight: 145 kg (net). | Chair: 1560 mm (L) × 720 mm (W) × 500–1050 mm (H, motorized). Base footprint: Ø 680 mm with enhanced stability ring. Weight: 168 kg (net) with composite reinforcement. |
| Precision | Positional repeatability: ±1.5 mm across 12 programmable positions. Hydraulic lift system with analog control. | Positional repeatability: ±0.3 mm across 24 memory presets. Servo-driven linear actuators with digital feedback loop and touch-screen trajectory calibration. |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade polyurethane (antimicrobial). Armrests: Molded ABS with stainless steel inserts. | Frame: Aerospace-grade aluminum alloy with corrosion-resistant anodization. Upholstery: Seamless silicone composite with self-healing surface. Armrests: Carbon-fiber reinforced polymer with integrated haptic controls. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC). | Full CE + FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (4th Ed), IEC 60601-2-57 (Photobiological Safety), UL 60601-1 (North America). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Strategic Imperative: China remains the dominant global hub for dental equipment manufacturing (75% market share, 2026 Global Dental Tech Report). However, supply chain volatility, regulatory tightening (EU MDR 2026 updates, FDA AI compliance), and quality inconsistencies necessitate a structured sourcing protocol. This guide outlines critical verification and negotiation steps for risk-mitigated procurement.
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable)
Counterfeit certifications account for 32% of dental equipment failures in emerging markets (2025 WHO Dental Safety Report). Implement this 4-point verification protocol:
| Verification Step | Technical Requirements | Red Flags |
|---|---|---|
| Document Authenticity | Request original ISO 13485:2016 & CE MDR 2017/745 certificates. Cross-check certificate numbers via: – EU EUDAMED (CE) – ANATEL database (Brazil) – SAMDRA portal (Saudi Arabia) – FDA Establishment Registration (for US-bound goods) |
Certificates issued by non-accredited bodies (e.g., “ISO Center of China”), PDF-only copies without wet-ink stamps, mismatched facility addresses |
| Product-Specific Coverage | Confirm certification scope EXPLICITLY lists: – Dental chairs (ISO 9917) – CBCT (IEC 60601-2-44) – Autoclaves (EN 13060) *Note: Intraoral scanners require separate IEC 60601-1-2 for EMC* |
Generic “medical device” coverage without model-specific annexes, expired certificates (>12 months) |
| Factory Audit Trail | Demand latest TÜV SÜD/BSI audit report (within 6 months). Verify: – Raw material traceability (steel alloys for chairs) – Sterilization validation records (autoclaves) – Radiation safety logs (CBCT) |
Refusal to share audit reports, reports not mentioning specific product lines |
| Post-Market Surveillance | Require evidence of: – Vigilance reporting system (MDR Article 83) – Field safety corrective action (FSCA) history – Cybersecurity protocols (for AI-powered scanners/CBCT) |
No documented PMS system, unexplained product recalls |
Step 2: Strategic MOQ Negotiation Framework
2026 market dynamics require flexible volume strategies. Avoid blanket minimums:
| Product Category | Standard MOQ (China) | 2026 Negotiation Levers | Carejoy Advantage |
|---|---|---|---|
| Dental Chairs | 5-10 units | Negotiate 3-unit MOQ for: – Clinics: Bundle with 2x autoclaves – Distributors: Commit to 12-month rolling forecast |
1-unit MOQ for OEM chairs with Carejoy’s modular platform (19 years tooling library) |
| Intraoral Scanners | 10 units | Reduce to 5 units with: – Prepayment of 50% – Firmware customization commitment |
Zero MOQ for Carejoy’s CJ-Scan Pro with distributor-branded UI (ODM) |
| CBCT Units | 2-3 units | Waive MOQ for: – Service contract prepayment (3 years) – Co-marketing agreements |
No MOQ with Carejoy’s container-load optimization (shared shipments) |
| Autoclaves | 20 units | Negotiate 10 units for: – Clinics: Full sterilization suite order – Distributors: Regional demo unit program |
5-unit MOQ for CJ-Steri series (in-stock components) |
Step 3: Optimized Shipping & Logistics (DDP vs. FOB 2026)
With 2026 IMO 2020 sulfur cap enforcement and port congestion risks, choose terms strategically:
| Term | 2026 Risk Allocation | When to Use | Cost Impact |
|---|---|---|---|
| FOB Shanghai | Clinic/Distributor bears: – Ocean freight volatility (2026 avg: $8,200/40ft) – US Section 301 tariffs (25% on dental chairs) – Destination port demurrage (avg. 7.2 days delay) |
For: – Large distributors with freight management teams – Orders >$150,000 (economies of scale) |
12-18% lower base price but 22-30% higher total landed cost risk |
| DDP (Delivered Duty Paid) | Supplier bears: – All freight/risk to clinic door – Customs clearance – 2026 EU carbon tax (CBAM) |
For: – Clinics without logistics expertise – First-time importers – Urgent replacement orders |
15-20% higher invoice price but 25-35% lower total cost predictability |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing:
- 19-Year Compliance Track Record: Unbroken ISO 13485 certification since 2007; CE MDR-compliant factory audit reports available upon NDA
- MOQ Innovation: Industry’s lowest volume thresholds via shared production lines (e.g., 1 dental chair MOQ with OEM)
- DDP Specialization: In-house logistics arm manages 92% of shipments DDP with price-locked 2026 freight contracts
- Product Integrity: Vertical integration (owns steel fabrication, PCB assembly) ensures component traceability
For Verified Supply Chain Access:
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Factory Direct)
📧 [email protected]
📱 WhatsApp: +86 15951276160 (24/7 English-speaking support)
Reference “2026 Guide” for priority factory audit scheduling
2026 Sourcing Imperative
China’s dental manufacturing ecosystem has matured beyond cost arbitrage. Success requires:
✅ Regulatory-first supplier vetting (beyond certificate screenshots)
✅ Dynamic MOQ strategies tied to value-add services
✅ DDP-focused logistics to mitigate 2026 tariff/carbon cost volatility
Partner with manufacturers demonstrating verifiable compliance infrastructure and supply chain transparency – not just transactional pricing. Shanghai Carejoy’s 19-year export history provides the operational backbone for risk-optimized procurement in an increasingly complex global landscape.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Equipment Focus: Dental Imaging Units (Panoramic & CBCT Systems)
Frequently Asked Questions: Procurement in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental imaging equipment in 2026? | Most modern panoramic and CBCT units operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In 2026, ensure compatibility with local grid specifications and consider units with built-in voltage stabilization, especially in regions with inconsistent power supply. Always verify the exact voltage, frequency (50/60 Hz), and amperage requirements in the technical datasheet prior to installation. |
| 2. Are spare parts for dental imaging systems readily available post-purchase, and what is the typical lead time? | Reputable manufacturers and certified distributors maintain global spare parts inventories for critical components such as X-ray tubes, sensors, gantry motors, and control boards. In 2026, leading brands offer guaranteed parts availability for a minimum of 7–10 years post-discontinuation. Average lead time for in-stock components is 3–7 business days; expedited shipping options are available. Always confirm parts support and service-level agreements (SLAs) before procurement. |
| 3. What does the installation process entail, and is on-site technical support included? | Installation of dental imaging units requires certified biomedical engineers and typically includes site preparation verification, hardware assembly, radiation safety testing, software configuration, and DICOM integration. In 2026, most premium suppliers include complimentary on-site installation and calibration as part of the purchase agreement. Remote connectivity for pre-installation assessment and post-installation diagnostics is now standard for efficient deployment. |
| 4. What is covered under the standard warranty, and are extended warranty options available? | The standard warranty for dental imaging equipment in 2026 typically covers parts and labor for 24 months, including the X-ray generator, detector, mechanical components, and software defects. Extended warranties up to 5 years are available and highly recommended, often including preventive maintenance visits, priority response, and predictive diagnostics via IoT-enabled monitoring systems. Review warranty terms for exclusions such as accidental damage or improper usage. |
| 5. How are software updates and service support managed during the warranty period? | During the warranty period, manufacturers provide free software updates, security patches, and remote diagnostic support via cloud-connected platforms. In 2026, AI-driven support systems enable proactive issue detection and resolution. Regular firmware updates enhance image quality, workflow efficiency, and compliance with evolving regulatory standards (e.g., GDPR, FDA 510(k)). Ensure your unit supports over-the-air (OTA) updates and is integrated with the vendor’s service network. |
Need a Quote for Dental Equipment Name?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


