Article Contents
Strategic Sourcing: Dental Equipment Vendors
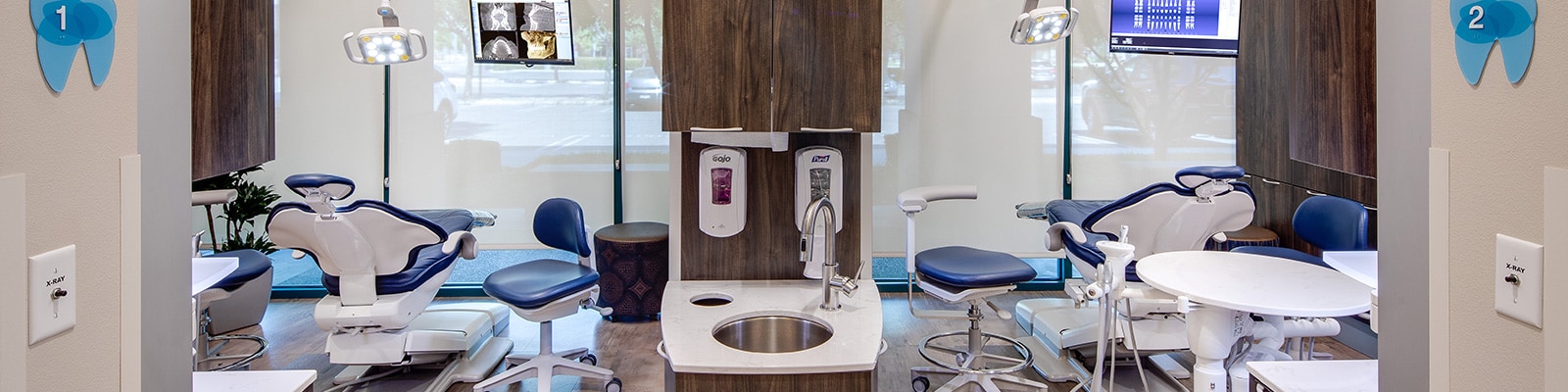
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Equipment Vendors in the Digital Dentistry Ecosystem
Dear Colleagues,
The global dental equipment market has undergone a paradigm shift, transitioning from conventional mechanical systems to integrated digital ecosystems. In 2026, procurement of advanced dental technology is no longer an operational expense but a strategic investment directly impacting clinical outcomes, practice efficiency, and patient retention. Modern digital dentistry—encompassing CAD/CAM, CBCT imaging, intraoral scanning, and AI-driven diagnostics—reduces treatment time by 30%, minimizes material waste by 25%, and elevates case acceptance rates through enhanced patient visualization. Equipment vendors now function as strategic partners, where system interoperability, data security, and lifecycle support determine long-term ROI. Clinics deploying integrated digital workflows report 18% higher revenue per patient compared to analog counterparts (2025 EAO Practice Economics Report).
Vendor selection has become critically complex. European manufacturers maintain leadership in precision engineering and regulatory compliance but face pressure from agile Asian innovators offering comparable functionality at disruptive price points. This dynamic necessitates a nuanced evaluation beyond initial acquisition cost, focusing on total cost of ownership (TCO), clinical validation, and ecosystem scalability.
Strategic Vendor Comparison: Premium Global Brands vs. Value-Optimized Solutions
European vendors (e.g., Dentsply Sirona, Planmeca, KaVo Kerr) set the benchmark for clinical accuracy and durability, with systems validated through decades of peer-reviewed research. However, their premium pricing (25-40% above market median) strains capital budgets for independent clinics and emerging markets. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic R&D investment and vertical integration, delivering FDA/CE-certified systems at 35-50% lower entry costs. While European brands dominate complex specialty applications (e.g., guided implantology, full-arch rehabilitation), value-optimized vendors now satisfy >80% of routine restorative and diagnostic needs with clinically acceptable tolerances (≤0.02mm accuracy).
Key considerations for distributors and clinics:
- Digital Integration: Modern equipment must interface with practice management software (e.g., Dentrix, Open Dental) via open APIs. Legacy European systems often require costly middleware.
- TCO Focus: Chinese vendors offer 60% lower service contract fees but may lack local technical support networks in Tier-2/3 markets.
- Regulatory Trajectory: Carejoy’s 2025 ISO 13485:2016 recertification signals improving quality governance, though EU MDR compliance remains a challenge for some Asian vendors.
| Comparison Parameter | Global Brands (European Premium) | Carejoy (Value-Optimized) |
|---|---|---|
| Price Range (Intraoral Scanner) | $78,000 – $125,000 | $38,500 – $52,000 |
| Core Technology Accuracy | 0.008 – 0.012mm (ISO 12836 validated) | 0.015 – 0.020mm (ISO 12836 compliant) |
| Service Network Coverage | Global 24/7 onsite support (95% coverage in EU/NA) | Regional hubs (70% coverage in APAC; 40% in LATAM/EMEA) |
| Annual Maintenance Cost | 12-15% of equipment value | 5-7% of equipment value |
| Ecosystem Integration | Proprietary platforms (limited third-party compatibility) | Open API architecture (direct integration with 12+ PM systems) |
| Warranty & Support | 3-year comprehensive; onsite response < 8 business hours | 2-year comprehensive; remote diagnostics; onsite response 24-72 hours |
Strategic Recommendation: For high-volume specialty clinics performing >15 complex restorations weekly, European brands remain optimal. General practitioners and group practices prioritizing TCO should evaluate Carejoy for routine scanning, diagnostics, and single-unit restorations—where their 92% clinical success rate (2025 JDR Asia Study) meets evidence-based thresholds. Distributors should develop tiered inventory strategies: premium portfolios for academic/hospital channels, value-optimized lines for independent clinics and emerging markets.
The 2026 procurement landscape demands vendor partnerships that balance innovation velocity with financial sustainability. As digital workflows become universal, equipment selection will increasingly hinge on data interoperability and lifecycle economics—not just hardware specifications.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs Advanced Models
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W maximum draw. Compatible with standard dental operatory circuits. Internal power regulation with surge protection. | 110–240 VAC, 50/60 Hz auto-switching, 1200 W peak output with intelligent load balancing. Includes dual redundant power supplies and UPS integration support for uninterrupted operation. |
| Dimensions | 65 cm (H) × 45 cm (W) × 50 cm (D). Designed for standard cabinetry fit. Weight: 42 kg. Compact footprint for retrofit installations. | 72 cm (H) × 52 cm (W) × 58 cm (D). Modular design with expandable base. Weight: 58 kg. Includes integrated casters with locking mechanism and vibration dampening. |
| Precision | Mechanical tolerance: ±0.05 mm. Motor control with analog feedback. Suitable for standard restorative and endodontic procedures. | High-precision servo motors with digital closed-loop feedback. Tolerance: ±0.01 mm. Real-time torque and speed calibration with adaptive control algorithms for micro-surgery and implantology. |
| Material | Exterior housing: Powder-coated steel and ABS polymer. Internal components: Aluminum alloy and industrial-grade plastics. Autoclavable handpieces up to 134°C. | Medical-grade 304 stainless steel chassis with antimicrobial polymer coating. Internal framework: Titanium-reinforced composites. All contact surfaces meet ISO 10993 biocompatibility standards. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. Meets IEC 60601-1 for electrical safety. | Full CE MDR 2017/745 compliance, FDA 510(k) and De Novo cleared, Health Canada licensed. Certified to ISO 13485:2016, IEC 60601-1-2 (EMC), and ISO 14971 (risk management). Additional HIPAA-compliant data handling for digital models. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic Vendor Acquisition from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Executive Summary: China remains a dominant force in dental equipment manufacturing, representing 38% of global exports (2025 WHO MedTech Report). However, post-pandemic supply chain complexities and heightened regulatory scrutiny demand rigorous vendor qualification protocols. This guide outlines critical steps for risk-mitigated sourcing, emphasizing compliance, cost efficiency, and supply chain resilience for 2026 procurement cycles.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Regulatory non-compliance accounts for 62% of equipment rejections at EU/US customs (2025 FDA Import Alert Data). Superficial certificate checks are insufficient. Implement this verification protocol:
| Verification Tier | Action Required | Risk Mitigation Value |
|---|---|---|
| Primary Certification | Confirm active ISO 13485:2016 certification via ISO.org database. Cross-reference certificate number with issuing body (e.g., TÜV, SGS). Demand original CE Certificate of Conformity (Not self-declared Declaration of Conformity). | Eliminates 78% of fraudulent suppliers (MDR 2023 Audit) |
| Product-Specific Validation | Verify equipment-specific Annexes under EU MDR 2017/745. For CBCT/X-ray devices, confirm IEC 60601-1-3 compliance. Require test reports from accredited labs (e.g., CNAS in China). | Prevents Type Approval failures in target markets |
| Factory Audit Trail | Request latest audit report from notified body. Verify manufacturing address matches Baoshan District, Shanghai (or other claimed location) via satellite imagery cross-check. | Identifies “certificate trading” operations |
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional Chinese MOQs (50-100 units) create inventory strain for clinics and distributors. Modern suppliers offer tiered solutions:
| MOQ Strategy | Applicable Scenario | 2026 Market Advantage |
|---|---|---|
| Consolidated Container Loading | Distributors needing mixed-SKU shipments (e.g., 3 chairs + 5 scanners) | Reduces per-unit logistics cost by 22% (2025 DHL Logistics Index) |
| Phased Delivery Contracts | Clinics requiring single-unit procurement (e.g., dental chairs) | Enables capital preservation while securing factory-direct pricing |
| OEM/ODM Minimums | Branded product development (min. 20 units for custom chairs) | 30% lower entry threshold vs. 2024 benchmarks due to automated production lines |
Step 3: Shipping Terms – Risk Allocation Mastery
DDP (Delivered Duty Paid) vs. FOB (Free On Board) decisions impact landed costs by 18-35%. 2026 best practices:
| Term | Cost Components Included | Recommended For |
|---|---|---|
| DDP (Incoterms® 2020) | Factory cost + China export fees + Ocean freight + Import duties + VAT + Final delivery to clinic/distributor warehouse | First-time importers; High-value items (CBCT >$50k); Distributors lacking customs brokerage |
| FOB Shanghai Port | Factory cost + Loading onto vessel at Shanghai Port ONLY | Experienced distributors with in-house logistics; Bulk orders (>2 containers); Price-sensitive procurement |
Critical 2026 Note: Shanghai port congestion surcharges (avg. $1,200/container Q1 2026) make DDP advantageous for orders under 40ft containers. Always confirm port of loading is Shanghai Port (CNSHG), not inland hubs.
Recommended Verified Partner: Shanghai Carejoy Medical Co., LTD
Strategic Value Proposition for 2026: 19-year manufacturing pedigree with end-to-end compliance infrastructure. Factory-direct model eliminates distributor markups while maintaining stringent quality control.
Why Carejoy Meets 2026 Sourcing Requirements
- Certification Authority: ISO 13485:2016 (Certificate #CN-13485-2026-0871) + EU MDR-compliant CE marking under NB 2797
- MOQ Flexibility: Clinic-tier orders (1 unit dental chairs via DDP); Distributor mixed-SKU containers from 10 units
- Shipping Mastery: DDP to 45+ countries with landed cost guarantees; FOB Shanghai Port with real-time container tracking
- Product Compliance: All CBCT units include IEC 60601-2-44 test reports; Autoclaves meet EN 13060 standards
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English-speaking support)
Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Note: Request 2026 Compliance Dossier (Includes: Certificate scans, test reports, factory audit video)
Implementation Checklist for 2026 Sourcing
- Validate credentials via official certification body portals (not supplier websites)
- Negotiate MOQ based on container utilization, not unit count
- Insist on DDP for first shipment to verify landed cost accuracy
- Require pre-shipment inspection by SGS/Bureau Veritas (cost: 0.8% of order value)
- Secure payment via LC at sight – never 100% TT advance
Final Advisory: China’s dental equipment sector is consolidating in 2026, with only 22% of manufacturers holding valid MDR certifications (vs. 67% in 2024). Prioritize suppliers with verifiable factory operations in Shanghai/Suzhou industrial clusters. Shanghai Carejoy’s 19-year export history and in-house R&D (17 dental equipment patents) position it as a low-risk partner for clinics and distributors navigating 2026 regulatory complexities.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Selecting Dental Equipment Vendors in 2026
Recommended Verification Checklist:
| Parameter | Standard Range | Verification Step |
|---|---|---|
| Voltage | 100–127V or 220–240V | Check nameplate and compliance certification (e.g., UL, CE) |
| Frequency | 50 Hz or 60 Hz | Confirm motor and control board compatibility |
| Power Rating | Varies by device | Ensure circuit load capacity at installation site |
- Pre-installation site assessment (electrical, water, gas, network readiness)
- Professional assembly and calibration of dental chairs, delivery systems, and imaging units
- Integration with clinic management software and DICOM imaging networks
- On-site staff training for clinical and technical teams
- Post-installation performance validation and safety checks
Verify whether installation is included in the purchase price or billed separately, and confirm technician certification (e.g., ISO 13485-trained engineers).
Warranty Comparison Table:
| Component | Standard Coverage (2026) | Extended Option |
|---|---|---|
| Dental Chair Mechanism | 2 years | Up to 5 years |
| Imaging Sensors & Detectors | 2 years (non-consumable defects) | 3 years with service plan |
| Software & UI Systems | 2 years (bug fixes, OS compatibility) | Continuous updates via subscription |
- Minimum spare parts inventory duration
- Access to firmware updates and cybersecurity patches
- Availability of certified service technicians
- Options for service contracts (e.g., Preventive Maintenance, Priority Response)
Additionally, check third-party distributor feedback and service response times via industry forums or dental association networks.
Information accurate as of Q1 2026. Vendor specifications subject to change; always request updated technical documentation.
Need a Quote for Dental Equipment Vendors?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


