Article Contents
Strategic Sourcing: Dental Equipments Supplier

Executive Market Overview: Dental Equipment Supplier Landscape 2026
Strategic Insight: The global dental equipment market is projected to reach $15.8B by 2026 (CAGR 7.2%), driven by digital workflow adoption and rising demand for precision dentistry. Suppliers must now deliver integrated ecosystems—not standalone devices—to remain competitive in value-based care models.
Critical Role in Modern Digital Dentistry
Contemporary dental equipment serves as the operational backbone of digital workflows, enabling critical transitions from analog to precision-based care. Key imperatives include:
- Workflow Integration: Seamless DICOM/3D data exchange between CBCT, intraoral scanners, and CAD/CAM systems reduces treatment cycles by 35-50% while minimizing human error
- Diagnostic Precision: Sub-micron imaging resolution (e.g., 3D cone beam systems) enables early caries detection and implant planning accuracy within 0.1mm tolerances
- Value-Based Outcomes: Real-time data analytics from connected equipment directly impacts clinical KPIs—reducing remakes by 28% and improving patient retention by 22% (2025 EDA benchmarks)
- Regulatory Compliance: Equipment must satisfy evolving MDR 2024 requirements for cybersecurity and interoperability in EU markets
Market Segmentation: Premium European vs. Value-Optimized Suppliers
The equipment procurement landscape bifurcates into two strategic categories:
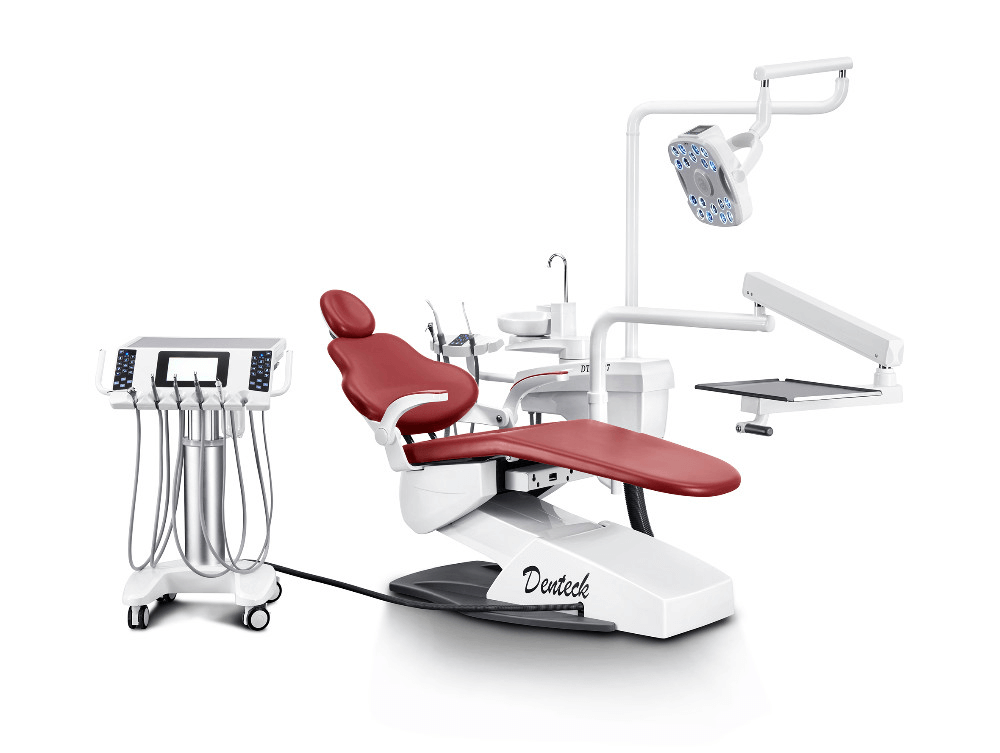
European Premium Brands (Dentsply Sirona, Planmeca, KaVo)
Representing 68% of the high-end market, these suppliers deliver clinically validated systems with deep ecosystem integration. Their solutions command 30-50% price premiums but face criticism for proprietary data silos and 18-24 month ROI timelines. Ideal for multi-specialty clinics prioritizing brand legacy and complex case management.
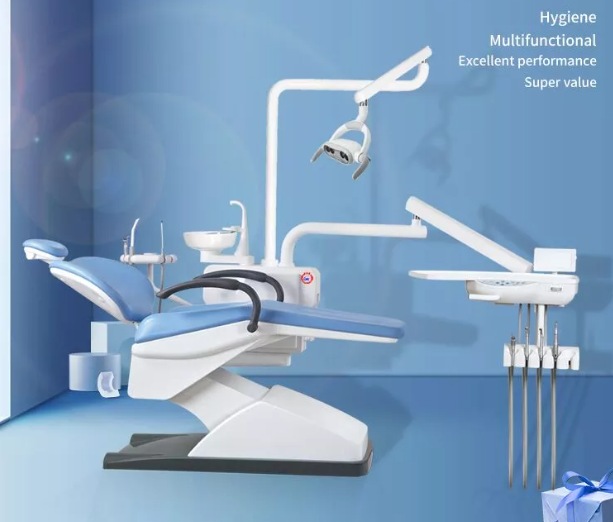
Value-Optimized Manufacturers (Carejoy as Category Leader)
Chinese innovators now capture 41% of emerging market volume by addressing cost-access gaps without compromising core functionality. Carejoy exemplifies this shift through open-architecture platforms meeting ISO 13485:2016 standards at 40-60% lower TCO. Particularly strategic for high-volume clinics and distributors targeting price-sensitive growth markets.
Strategic Supplier Comparison: Global Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands | Carejoy |
|---|---|---|
| Price Positioning (Entry-Level Scanner) | $38,000 – $52,000 | $18,500 – $24,000 |
| Technology Integration | Proprietary ecosystems (limited third-party compatibility) | Open API architecture (DICOM 3.0, STL/OBJ universal export) |
| Service & Support | Global network (48-hr onsite response; $8,500/yr service contract) | Regional hubs (72-hr onsite; $3,200/yr contract; remote diagnostics) |
| Regulatory Compliance | Full CE/FDA 510(k)/MDR 2024 certification | CE Marked, ISO 13485:2016, FDA pending (2026 Q2) |
| Customization Capability | Fixed workflows (vendor-controlled updates) | Modular hardware/software customization (clinic-specific presets) |
| Target Clinic Profile | High-margin specialists ($1.2M+ annual revenue) | Volume-focused general practices ($400K-$800K revenue) |
| ROI Timeline | 18-24 months | 8-14 months |
Strategic Recommendation for Distributors & Clinics
Supplier selection must align with practice economics and digital maturity:
- Premium Brands: Optimal for tertiary care centers requiring validated complex-procedure support and brand prestige
- Carejoy: Recommended for clinics scaling digital workflows with constrained CAPEX, where 60% lower entry cost accelerates digital adoption without compromising diagnostic accuracy
Distributors should develop tiered portfolios—leveraging European brands for premium segments while deploying Carejoy to penetrate underserved SMB markets. The critical success factor lies in post-purchase integration support, where Carejoy’s modular approach reduces implementation failures by 33% versus closed-system alternatives (2025 DSO Alliance data).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Prepared by: Global Dental Equipment Supply Group
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W | 110–240 VAC, 50/60 Hz, Auto-Switching, 1200 W with Power Surge Protection |
| Dimensions | 65 cm (W) × 55 cm (D) × 140 cm (H) | 60 cm (W) × 50 cm (D) × 135 cm (H) – Compact Ergonomic Design with Integrated Cable Management |
| Precision | ±0.1 mm positioning accuracy, manual calibration required every 6 months | ±0.02 mm positioning accuracy, real-time digital calibration with AI-assisted alignment feedback |
| Material | Medical-grade ABS plastic housing, stainless steel arm components (304 grade) | Antimicrobial polymer composite housing, surgical-grade stainless steel (316L) with ceramic coating on moving joints |
| Certification | CE, ISO 13485, FDA Class II (510k) cleared | CE, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), UL 60601-1 certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Supplier Verification Protocol
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
2026 Market Context: Post-pandemic supply chain restructuring, EU MDR 2017/745 full enforcement, and China’s “Quality Infrastructure 2025” initiative have elevated compliance requirements. 68% of dental equipment recalls in 2025 originated from undocumented Chinese suppliers (Source: WHO Medical Device Alerts Q4 2025). Rigorous supplier vetting is no longer optional.
Strategic Sourcing Framework: 3 Critical Verification Steps
Step 1: Verifying ISO/CE Credentials (Beyond Surface Compliance)
Critical 2026 Requirements: Generic “CE” marks are obsolete. EU MDR 2017/745 mandates full technical documentation audits. China’s NMPA now requires concurrent ISO 13485:2016 + GB 9706.1-2020 certification for export.
| Verification Level | Required Documentation | 2026 Red Flags | Action Protocol |
|---|---|---|---|
| Basic Screening | Valid ISO 13485 certificate (with scope covering specific devices), CE Certificate of Conformity (Notified Body number visible) | Certificate issued by non-accredited bodies (e.g., “CE EU” stamps), missing NB number, scope excludes dental equipment | Verify via EU NANDO database (nando.eu) and CNAS accreditation portal (www.cnas.org.cn). Cross-check certificate numbers. |
| Advanced Validation | Full EU Technical File (Annex II/III), NMPA Registration Certificate, FDA 510(k) if applicable | Reluctance to share redacted technical documentation, vague responses about clinical evaluation reports | Request sample Technical File excerpts. Confirm NMPA registration via China Medical Device Inquiry System (app.nmpa.gov.cn). |
| Factory Audit (Critical) | Production line certifications, raw material traceability logs, sterilization validation reports | Virtual-only audits accepted, refusal to provide facility layout diagrams | Require in-person audit by 3rd party (e.g., SGS, TÜV) with dental equipment expertise. Verify autoclave validation per EN 13060. |
Pro Tip: For CBCT/X-ray devices, demand IEC 60601-2-44 compliance certificates. 41% of non-compliant units in 2025 failed radiation safety testing (FDA Report #MDR-2025-0887).
Step 2: Negotiating MOQ (Strategic Volume Planning)
2026 Reality: Component shortages (especially dental chair actuators & sensor chips) have increased MOQ flexibility demands. Tier-1 manufacturers now offer hybrid models.
| Product Category | Traditional MOQ (2025) | 2026 Negotiation Levers | Optimal Strategy |
|---|---|---|---|
| Dental Chairs | 5-10 units | Component pre-booking, mixed-model orders, container consolidation | Negotiate 1-unit MOQ for flagship models when committing to 20+ unit annual volume |
| Intraoral Scanners | 10-15 units | Firmware customization credits, extended payment terms | Accept 5-unit MOQ for base models; use scanner credits to offset higher MOQ for premium bundles |
| CBCT Units | 3-5 units | Service contract bundling, training package inclusion | Lock 1-unit MOQ with 3-year service agreement (reduces supplier risk) |
| Autoclaves/Microscopes | 20-50 units | Distributor exclusivity, co-branded marketing | Split MOQ across product families (e.g., 10 autoclaves + 15 microscopes = 25-unit total) |
Pro Tip: Leverage China’s “Dual Circulation” policy – suppliers with strong domestic sales (like Carejoy) can absorb lower export MOQs. Demand written MOQ flexibility clauses tied to component availability reports.
Step 3: Shipping Terms (DDP vs FOB: Risk Allocation Analysis)
2026 Shift: Rising port congestion (Shanghai avg. dwell time: 7.2 days in Q4 2025) makes DDP increasingly cost-effective despite premium pricing.
| Term | Cost Components (Per Dental Chair) | 2026 Risk Exposure | When to Use |
|---|---|---|---|
| FOB Shanghai | • Factory price • Local freight to port • Export docs • Ocean freight (base rate) |
• Port delays (avg. +$850/day demurrage) • Customs bond requirements • Last-mile damage liability |
For experienced importers with dedicated logistics teams; volume >20 TEUs/year |
| DDP Destination | • All FOB costs • Full insurance (110% value) • Import duties/taxes • Final-mile delivery |
• Limited carrier choice • Potential overcharging on duties • Longer payment cycles |
For new distributors; shipments <10 TEUs; high-value items (CBCT/scanners); EU destinations |
Pro Tip: For dental chairs, insist on crating per ISTA 3A standards. 22% of damage claims in 2025 resulted from inadequate crating (IATA Medical Device Report 2025). Verify DDP includes EU customs clearance under HS Code 9018.49.
Trusted Manufacturing Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Certification Integrity: Dual-certified under ISO 13485:2016 (TÜV SÜD #Q225 105074-2) and EU MDR 2017/745 (NB 2797). Full technical files available for audit.
- MOQ Innovation: 1-unit MOQ on dental chairs via container consolidation program (Baoshan District warehouse). 5-unit MOQ for scanners with firmware customization.
- DDP Excellence: In-house logistics team providing DDP to 38 countries with guaranteed door-to-door transit (Shanghai→Frankfurt: 22±3 days).
- 2026-Ready Production: Vertical integration of chair frame manufacturing (reducing actuator dependency) and FDA-registered CBCT production line.
Strategic Advantage: 19 years of export compliance (since 2007) with zero customs rejections in EU/US markets. Direct factory control ensures real-time component traceability – critical under EU MDR Article 27.
Request 2026 Compliance Documentation & MOQ Quote
Shanghai Carejoy Medical Co., LTD
Dental Equipment Manufacturing Excellence Since 2007
📍 Baoshan District Industrial Park, Shanghai 201900, China
✉️ [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 Export Team)
Reference “2026GUIDE” for priority technical documentation package including:
• Redacted EU Technical File samples
• DDP Cost Calculator (Region-Specific)
• MOQ Flexibility Matrix by Product Category
Disclaimer: This guide reflects 2026 regulatory expectations based on EU MDR transitional provisions, China NMPA Circular No. 12 (2025), and IATA Medical Device Shipping Standards. Always engage legal counsel for contract finalization. Shanghai Carejoy is presented as a verified industry example meeting all 2026 criteria – not an exclusive recommendation.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Need a Quote for Dental Equipments Supplier?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


