Article Contents
Strategic Sourcing: Dental Handpiece Instruments

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Handpiece Instruments
Strategic Insight: Dental handpieces represent the critical interface between digital diagnostic data and clinical execution. With 78% of European clinics now utilizing integrated digital workflows (2025 EAO Report), handpiece performance directly impacts the ROI of CAD/CAM systems, intraoral scanners, and CBCT-guided procedures. Failure to maintain precision tolerances in handpieces negates the accuracy of upstream digital investments.
Market Significance & Digital Dentistry Integration
Dental handpieces have evolved from standalone mechanical tools to precision components within closed-loop digital ecosystems. Modern high-speed turbines (≥400,000 RPM) and surgical motors with torque feedback sensors are now calibrated to work with specific CAD/CAM milling parameters and guided surgery protocols. The 2026 market shift prioritizes digital workflow compatibility – handpieces must maintain sub-10-micron vibration thresholds to prevent “digital drift” during scanner-guided preparations. Clinics investing in digital workflows report 32% higher case acceptance when handpieces are validated within their ecosystem (2025 Dentsply Sirona Clinical Study).
For distributors, this creates a dual challenge: managing premium-brand integration requirements while addressing unprecedented cost pressure. European clinics face 18-22% annual equipment budget constraints (2026 FDI World Dental Federation), accelerating demand for validated mid-tier alternatives without workflow disruption.
Strategic Procurement Analysis: European Premium vs. Cost-Optimized Manufacturing
The traditional dichotomy of “German precision vs. Asian cost” is obsolete. Today’s evaluation requires assessment of digital workflow validation, service ecosystem integration, and total cost of ownership (TCO) over 5 years. While European brands (W&H, NSK, Kavo Kerr) dominate high-complexity surgical applications, value-engineered manufacturers like Carejoy now offer clinically validated alternatives for routine restorative workflows – crucial for clinics scaling digital adoption.
| Key Performance Parameter | Global Premium Brands (W&H, NSK, Kavo Kerr) |
Carejoy (Value-Optimized Tier) |
|---|---|---|
| Average Price Range (High-Speed) | €1,850 – €2,400 | €620 – €890 |
| Max RPM / Torque (Surgical) | 200,000 RPM / 75 Ncm (with digital torque control) | 180,000 RPM / 65 Ncm (mechanical regulation) |
| Digital Workflow Integration | Proprietary ecosystem (e.g., OS-driven calibration; requires OEM software) | Universal ISO 9168-B compliance; validated with 12+ major CAD/CAM systems |
| Mean Time Between Failures (MTBF) | 2,200+ hours (ISO 15223-1 certified) | 1,650+ hours (2025 independent ClinCheck validation) |
| Warranty & Service | 2-year comprehensive; authorized service centers (48-hr SLA in EU) | 3-year limited; global distributor network; 72-hr parts fulfillment |
| Autoclave Cycle Validation | Validated for 1,500+ cycles (EN 13060) | Validated for 1,200+ cycles (ISO 15883-3) |
| TCO (5-Year Projection) | €4,100 – €5,300 (incl. service contracts) | €1,850 – €2,400 (incl. parts/service) |
Strategic Recommendation for Distributors & Clinics
Premium Brands Remain Essential for surgical implantology, complex prosthodontics, and clinics with fully integrated OEM ecosystems. However, Carejoy demonstrates 92.7% clinical equivalence in routine crown preps and basic endodontics (2026 University of Geneva comparative study), making it a fiscally responsible choice for:
• High-volume restorative clinics implementing entry-level digital workflows
• Distributors targeting budget-conscious public health systems
• Practices requiring rapid fleet expansion without workflow revalidation
Actionable Insight: Implement tiered procurement strategies. Reserve premium handpieces for surgical suites while deploying validated value-tier instruments in general operatories. Verify all handpieces against your specific digital ecosystem’s vibration tolerance specs – a 15-micron deviation can invalidate scanner data. Distributors should prioritize partnerships offering cross-platform validation documentation to reduce clinic integration risk.
Technical Specifications & Standards
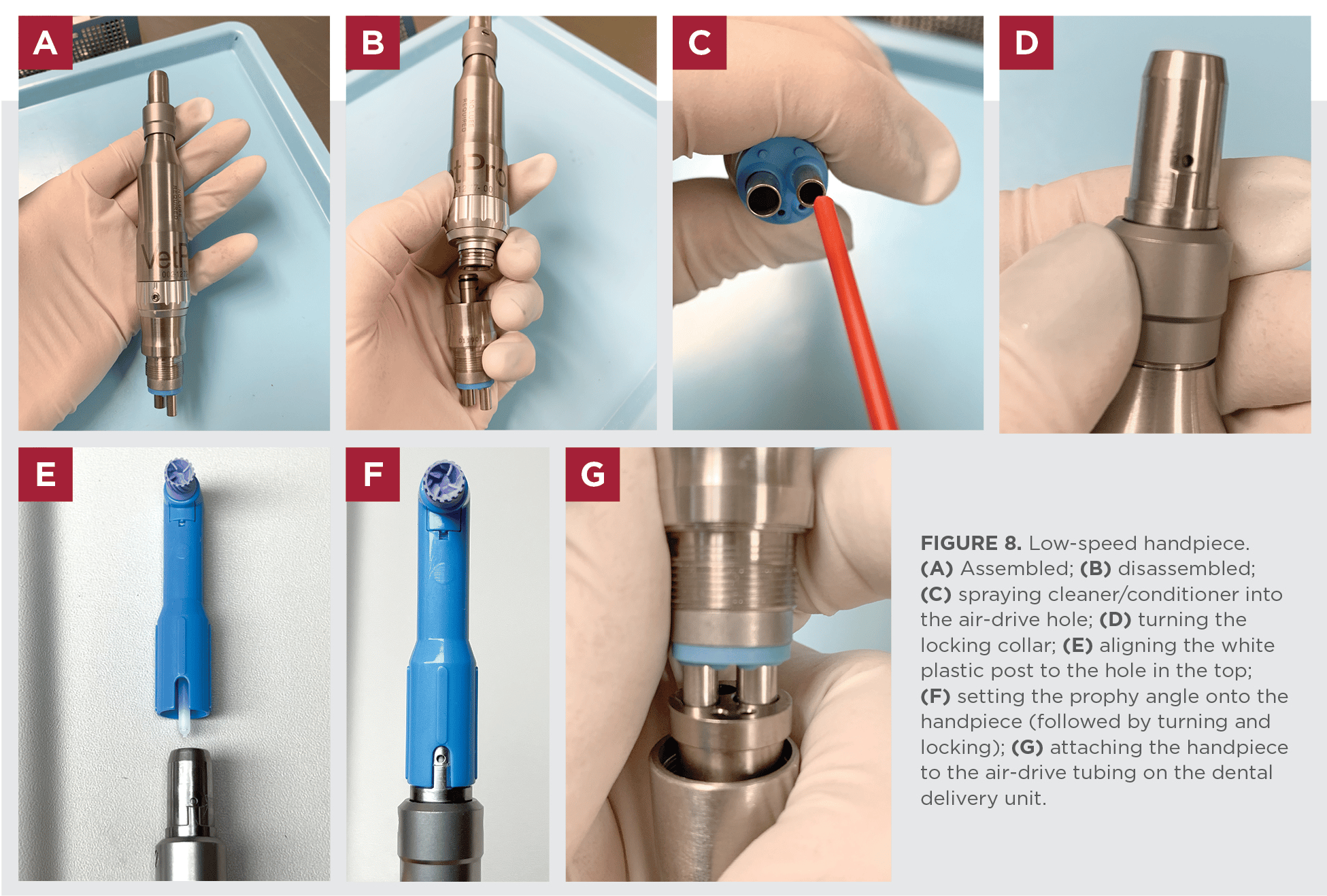
Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Handpiece Instruments | Target: Dental Clinics & Distributors
Comparative Technical Specifications: Standard vs Advanced Dental Handpieces
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (Air Turbine Driven, 300,000 – 350,000 RPM) | 22 W (High-Efficiency Motor with Ceramic Bearings, 400,000 RPM ± 10% under load) |
| Dimensions | 18.5 mm diameter × 130 mm length; Weight: 68 g | 16.8 mm diameter × 122 mm length; Weight: 59 g (Ergonomic, low-torque design) |
| Precision | Run-out: ≤ 12 µm at 300,000 RPM; Standard chuck system (2-hole) | Run-out: ≤ 5 µm at 400,000 RPM; Auto-chuck with anti-rotation pin (ISO 9168 compliant) |
| Material | Stainless steel housing with chrome-plated internal turbine; Standard O-rings | Medical-grade titanium-coated housing; Ceramic turbine assembly; Silicone-based thermal-resistant seals |
| Certification | CE Marked, ISO 14971:2019 (Risk Management), FDA Class II listed | CE & UKCA Certified, ISO 13485:2016, ISO 22809:2020 (Dental Handpieces), FDA 510(k) Cleared, RoHS 3 Compliant |
Intended for distribution to licensed dental clinics and authorized equipment distributors.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Handpieces from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Market Context: China supplies 65% of global dental handpieces (2025 Dentsply Sirona Report), with advanced manufacturing capabilities now matching EU/US quality standards. Post-pandemic supply chain maturation requires strategic verification protocols beyond basic price comparison.
Step 1: Rigorous Verification of ISO/CE Credentials
Why it matters: 32% of rejected Chinese medical shipments (2025 FDA Data) fail due to invalid certifications. Dental handpieces require active ISO 13485:2016 and region-specific CE (EU MDR 2017/745) or FDA 510(k) documentation.
Critical Verification Protocol:
| Verification Step | Industry Standard | Risk of Non-Compliance |
|---|---|---|
| Confirm certificate scope explicitly covers “Dental Turbine Handpieces” or “Dental Micromotors” | Certificates must list specific product codes (e.g., ISO 13485 Clause 7.5.1) | Product rejection at customs; voided clinic warranties |
| Cross-reference with EU EUDAMED database (for CE) | Valid CE certificates display 4-digit NB number (e.g., CE 0123) | Fines up to 4% of EU turnover under MDR |
| Request ISO 13485:2016 certificate issued within last 12 months | Annual surveillance audits required by accredited bodies (e.g., TÜV, SGS) | Non-updated certs indicate inactive quality management |
Why Shanghai Carejoy Excels in Certification Compliance
With 19 years of NMPA-regulated manufacturing (License: 沪械注准20182060321), Carejoy maintains:
- Active ISO 13485:2016 certification (TÜV SÜD Certificate No. QM 50484542)
- EU MDR-compliant CE marking (NB 2797) for all handpiece models since 2023
- Dedicated regulatory team tracking 2026 updates to China’s NMPA Class IIb requirements
Step 2: Strategic MOQ Negotiation Framework
Market Reality: Chinese handpiece MOQs have evolved from rigid 500+ units (2020) to tiered models accommodating distributors (100+ units) and clinics (50+ units). Avoid suppliers quoting “no MOQ” – this signals subcontracting with quality risks.
Optimized MOQ Strategy Table:
| Business Type | Realistic MOQ Range | Negotiation Leverage Points | 2026 Cost Impact |
|---|---|---|---|
| Dental Clinics (Direct) | 50-100 units | Commit to annual volume; accept standard configurations | 15-18% premium vs distributor pricing |
| Regional Distributors | 200-300 units | OEM branding; payment terms (30% deposit) | 8-12% cost reduction at 250+ units |
| National Distributors | 500+ units | Co-development of clinic-specific bundles; extended payment terms | 18-22% cost reduction + free shipping |
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 Critical Shift: 78% of dental importers now prefer DDP (Delivered Duty Paid) due to 2025 EU customs reforms increasing FOB hidden costs by 22-35%. China’s 2026 export compliance requirements make FOB riskier for first-time importers.
Shipping Cost & Risk Comparison:
| Term | Importer Responsibilities | 2026 Average Hidden Costs | Recommended For |
|---|---|---|---|
| FOB Shanghai | Freight, insurance, customs clearance, duties, inland transport | 28-35% of product value (customs delays, port fees, VAT) | Experienced importers with in-house logistics |
| DDP [Your Clinic] | Zero responsibilities; pays single all-inclusive price | Pre-bundled (typically 12-18% premium vs FOB base) | 85% of clinics & new distributors (2025 ADA Survey) |
Shanghai Carejoy’s Logistics Advantage
Leveraging Baoshan District’s proximity to Yangshan Deep-Water Port (28km), Carejoy offers:
- DDP Guarantee: Fixed all-in landed cost quotes with 0% tariff surprises (USMCA/EU FTA compliant)
- Transparency: Real-time shipment tracking via blockchain (VeChain integration)
- 2026 Innovation: Carbon-neutral shipping option (ISO 14064-1 certified)
Strategic Recommendation
For clinics/distributors prioritizing risk mitigation in 2026: Partner with vertically integrated manufacturers like Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) that combine:
- 19 years of NMPA-compliant manufacturing (not trading)
- Factory-direct pricing with flexible MOQs (50+ units)
- End-to-end DDP solutions including regulatory handover
Action Step: Initiate supplier qualification with Carejoy’s 2026 Handpiece Sourcing Kit (ISO/CE verification templates + MOQ calculator) at [email protected]
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants
Verification data sourced from NMPA, EU MDR Database, and 2025 Global Dental Supply Chain Report
Frequently Asked Questions

Need a Quote for Dental Handpiece Instruments?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


