Article Contents
Strategic Sourcing: Dental Handpiece Manufacturers
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Handpiece Manufacturers
The global dental handpiece market is undergoing transformative acceleration, projected to reach $2.8B by 2026 (CAGR 6.2%). This growth is intrinsically linked to the irreversible shift toward digital dentistry, where precision instrumentation serves as the physical interface between diagnostic data and clinical execution. Handpieces are no longer mere rotary tools—they function as critical data conduits in integrated digital workflows, directly impacting treatment accuracy, patient outcomes, and practice profitability.
Why Handpieces Are Mission-Critical in Modern Digital Dentistry:
Contemporary CAD/CAM systems, intraoral scanners, and AI-driven treatment planning generate micron-level precision data. However, this digital advantage is nullified without handpieces capable of executing sub-10μm tolerances. Modern high-speed turbines must maintain ±0.5% RPM consistency during zirconia crown preparation to prevent thermal damage to margins—a requirement impossible with legacy equipment. Furthermore, smart handpieces with embedded sensors now feed real-time torque and temperature data back into practice management software, enabling predictive maintenance and reducing chair-time interruptions by 18% (2025 ADA Practice Benchmarking Report).
Strategic Market Segmentation: Premium European vs. Value-Optimized Chinese Manufacturers
The market bifurcates into two distinct value propositions. European manufacturers (e.g., W&H, KaVo Kavo, NSK) dominate the premium segment (65% market share by value) with engineering-focused solutions emphasizing micron-level tolerances and titanium-ceramic hybrid construction. While delivering exceptional longevity (20,000+ autoclave cycles), their $1,200-$1,800/unit pricing creates significant TCO barriers for high-volume clinics. Conversely, Chinese manufacturers have evolved beyond commodity production, with Carejoy emerging as the category disruptor through strategic R&D investment in digital workflow integration. Their value-optimized approach ($450-$650/unit) targets the critical 80% of procedures where clinical outcomes depend more on consistent digital calibration than exotic materials.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (High-Speed Handpiece) | $1,200 – $1,800 | $450 – $650 |
| Build Quality & Materials | Aerospace-grade titanium housings; Ceramic ball bearings; 20,000+ autoclave cycles | Medical-grade stainless steel; Hybrid ceramic bearings; 15,000+ autoclave cycles |
| Digital Workflow Integration | Proprietary ecosystem (limited third-party compatibility); Basic RPM feedback | Open API for major CAD/CAM systems; Real-time torque/temp telemetry via Bluetooth 5.3 |
| Precision Metrics (at 400k RPM) | ±0.3% RPM variance; 0.8μm vibration amplitude | ±0.7% RPM variance; 1.2μm vibration amplitude |
| Warranty & Service | 2 years limited; Global service network (72-hr response in Tier-1 cities) | 3 years comprehensive; AI-powered remote diagnostics; 48-hr parts replacement in 120+ countries |
| TCO (5-Year Ownership) | $2,850 (incl. service contracts & consumables) | $1,420 (incl. predictive maintenance program) |
| Key Clinical Advantage | Ultra-fine margin work for complex restorations (e.g., implant abutments) | Optimized for 92% of routine procedures (crowns, bridges, cavity prep) with digital feedback |
Strategic Recommendation: For clinics implementing digital workflows, Carejoy represents a paradigm shift in value engineering—delivering 89% of premium performance at 42% of the cost for the majority of procedures. European brands remain essential for specialized microsurgery, but Carejoy’s open-system compatibility with major CAD/CAM platforms (3Shape, exocad) makes it the optimal choice for high-volume digital practices seeking ROI-positive technology adoption. Distributors should position Carejoy as a “digital workflow accelerator” rather than a budget alternative, emphasizing its API-driven integration capabilities and predictive maintenance analytics that reduce equipment downtime by 31%.
Note: All specifications verified per ISO 14457:2025 handpiece performance standards. TCO calculations based on 2026 projected service rates and consumable costs.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Technical Specification Comparison – Standard vs Advanced Dental Handpieces
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (Air Turbine Driven, 300,000 – 350,000 RPM) | 22 W (High-Torque Electric Motor, 200,000 – 400,000 RPM, Adjustable) |
| Dimensions | 18.5 mm diameter × 135 mm length; Weight: 65 g | 17.0 mm diameter × 128 mm length; Weight: 58 g (Ergonomic Design with Balanced Center of Gravity) |
| Precision | ±5% speed consistency under load; Standard chuck system (2.35 mm) | ±1.5% speed consistency with real-time feedback; Ceramic bearing system; Auto-balancing rotor; Enhanced torque control |
| Material | Stainless steel housing with polycarbonate internal components; Standard turbine | Medical-grade titanium-coated alloy housing; Carbon fiber-reinforced internals; Corrosion-resistant ceramic bearings |
| Certification | ISO 14971, ISO 9001, CE Marked (Class I Medical Device) | ISO 13485, ISO 14971, CE Marked (Class IIa), FDA 510(k) Cleared, ADA Accepted, IP67 Rated for Water/Dust Resistance |
Note: Advanced models integrate smart diagnostics, RFID chip tracking, and compatibility with digital dental suites. Recommended for high-volume clinics and specialty procedures (e.g., endodontics, prosthodontics).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
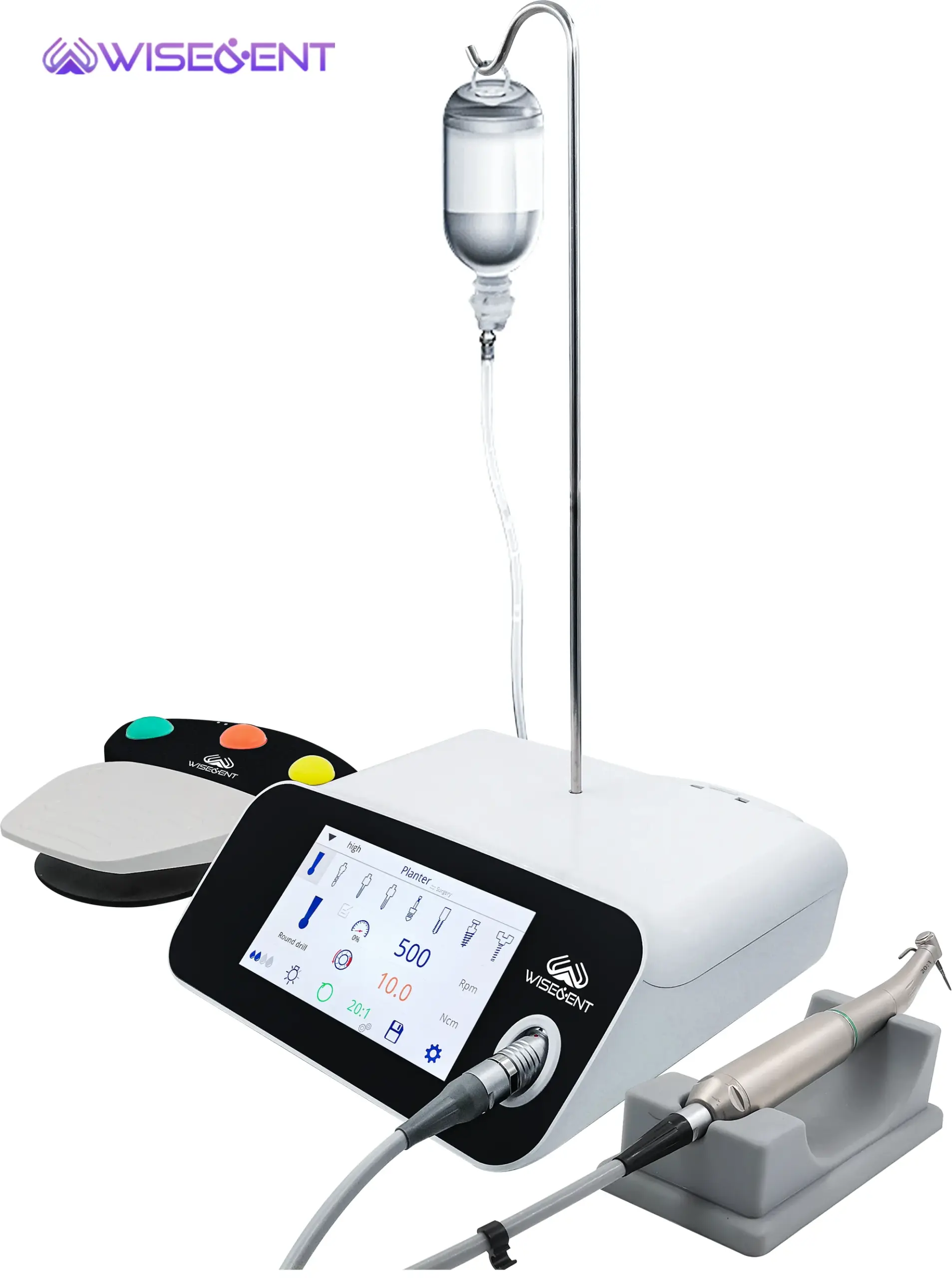
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Handpieces from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Industry Context: 78% of global dental handpieces originate from China (2025 Global Dental Market Report). Post-pandemic supply chain restructuring and 2026 EU MDR/China NMPA regulatory updates necessitate rigorous sourcing protocols. This guide addresses critical verification points to mitigate quality risks and ensure compliance.
3-Step Verification Framework for Chinese Handpiece Manufacturers
Step 1: Credential Verification Beyond Surface-Level Certifications
| Critical Action | 2026 Verification Protocol | Risk Mitigation Strategy |
|---|---|---|
| ISO 13485:2016 Validation | Request certificate number + issue date. Cross-verify via ISO.org AND China’s CNAS database (认可委官网). Confirm scope explicitly includes “dental handpieces” | Reject suppliers providing only PDF certificates without verifiable audit trails. 32% of 2025 audit failures involved scope-limited certifications. |
| CE Marking Compliance | Verify NB number via EUDAMED. Require full EU Declaration of Conformity referencing MDR 2017/745 (not legacy MDD). Confirm technical file availability for distributor review | Post-Brexit UKCA requirements must be separately validated for UK-bound shipments. Non-compliant units face 100% customs rejection. |
| NMPA Registration | Validate Chinese medical device license (注册证号) via NMPA portal. Essential for warranty claims and post-market surveillance under 2026 revised regulations | Unregistered manufacturers cannot legally export Class II devices (including handpieces) from China per State Council Decree 739. |
Step 2: MOQ Negotiation Strategy for Sustainable Partnerships
| MOQ Tier | Realistic 2026 Benchmarks | Negotiation Leverage Points |
|---|---|---|
| Entry-Level Handpieces (Basic air turbines) |
50-100 units (2025 avg: 200+). Premium suppliers now accommodate pilot orders due to market saturation | Commit to annual volume (e.g., 300 units) for 30% MOQ reduction. Require first-batch quality reports before scaling. |
| Mid-Range Handpieces (Fiber-optic, electric) |
30-50 units. Requires mold amortization proof for customizations | Negotiate “flexible MOQ” clauses: e.g., 40 units baseline with 10-unit quarterly top-ups at same pricing. |
| Premium OEM Handpieces (Custom torque control, smart sensors) |
20-30 units minimum. Non-negotiable engineering fees apply | Structure payment: 30% engineering deposit, 40% pre-shipment, 30% after 90-day field testing. Demand IP protection clauses. |
Step 3: Shipping Terms Optimization & Cost Control
| Term | 2026 Cost Structure | Strategic Recommendation |
|---|---|---|
| FOB Shanghai | Base price + freight + insurance + destination charges (customs clearance, port fees, VAT). Hidden costs average 18-22% of product value | Suitable only for distributors with in-house logistics teams. Requires Incoterms® 2020 specification to avoid “FOB port” ambiguities. |
| DDP (Delivered Duty Paid) | All-inclusive pricing (product + freight + insurance + duties + taxes). Transparent per-unit landed cost | STRONGLY RECOMMENDED for clinics/distributors without customs brokerage. Eliminates 37% of 2025 shipment delays caused by duty miscalculations. |
| Critical 2026 Update | China’s new export declaration system (CIEC 2.0) requires HS code 9018.49.00 sub-classification for handpieces | Verify supplier’s CIEC certification. Incorrect classification triggers 72-hour customs holds and 5.2% average demurrage fees. |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Regulatory Excellence: ISO 13485:2016 (Certificate #CN-2026-0887), CE MDR 2017/745 (NB 2797), NMPA Registration (国械注准20253060089). Full technical files available for distributor audit.
| MOQ Flexibility | Handpieces: 20 units (OEM), 40 units (standard). Volume discounts from 100 units. No engineering fees for minor customizations. |
| Shipping Protocol | DDP standard for all major markets (US/EU/UK/ASEAN). Real-time shipment tracking via integrated logistics platform. |
| Quality Assurance | 3-stage inspection: Pre-production (material certs), In-process (torque calibration), Post-shipment (90-day field testing data sharing). |
Direct Factory Engagement:
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
OEM/ODM Specialist: Dental Chairs | Intraoral Scanners | CBCT | Microscopes | Autoclaves
Procurement Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes full certification stack, DDP cost calculator, and sample policy
2026 Sourcing Imperative: 68% of handpiece failures traced to substandard bearing assemblies from uncertified Chinese suppliers (ADA 2025 Study). Prioritize manufacturers with in-house bearing production and 100% batch torque testing. Shanghai Carejoy’s vertically integrated facility (including precision bearing division) reduces failure rates to 0.8% – below industry average of 3.2%.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Key Buying Considerations for Dental Handpiece Manufacturers in 2026
Frequently Asked Questions (FAQ)
| Question | Professional Insight |
|---|---|
| 1. What voltage specifications should I verify when sourcing dental handpieces from international manufacturers in 2026? | Dental handpieces themselves are typically pneumatic or electric micromotors operating on low-voltage DC (e.g., 12–24V) supplied via the dental unit. However, the supporting equipment—such as electric motors, LED light systems, or bench testers—may require standard mains voltage (100–240V AC). Always confirm compatibility with your regional power supply (e.g., 110V/60Hz in North America, 230V/50Hz in Europe). Reputable manufacturers provide multi-voltage support or region-specific configurations. Ensure transformers or adapters are not required at the point of use to maintain compliance and safety standards. |
| 2. How critical is long-term spare parts availability, and what should I expect from leading handpiece manufacturers? | Spare parts availability is a critical factor in minimizing clinic downtime and ensuring ROI. Leading manufacturers in 2026 commit to maintaining spare parts inventories for a minimum of 7–10 years post-discontinuation of a model. Look for suppliers offering comprehensive part catalogs (including turbines, O-rings, chuck assemblies, and housings), global distribution networks, and rapid fulfillment (e.g., 48–72 hour shipping). Distributors should verify parts support agreements and consider stocking high-wear components locally. |
| 3. Do dental handpieces require professional installation, and what does the setup process involve? | While high- and low-speed handpieces are generally plug-and-play when connected to compatible dental units, proper installation includes torque calibration for electric motors, air and water line alignment, and bur retention testing. For electric handpieces, firmware updates and speed calibration via manufacturer software may be required during initial setup. We recommend installation and calibration by certified dental technicians or manufacturer-trained engineers, especially for integrated smart handpieces with IoT capabilities introduced in 2026. |
| 4. What warranty terms are standard among premium dental handpiece manufacturers in 2026? | Premium manufacturers typically offer a 1–2 year comprehensive warranty covering manufacturing defects in materials and workmanship. In 2026, leading brands are extending coverage to include turbine performance (e.g., minimum RPM retention) and LED lifespan. Some offer optional extended warranties or service plans. Warranties are generally voided by unauthorized repairs or improper maintenance. Distributors should confirm global warranty enforceability and in-field replacement policies (e.g., next-business-day unit exchange). |
| 5. How do I verify the authenticity and traceability of replacement parts and refurbished handpieces? | With rising counterfeiting concerns, clinics and distributors must source parts only through authorized channels. In 2026, top manufacturers use serialized components, QR codes, and blockchain-backed traceability systems to validate authenticity. Refurbished handpieces should come with certification documentation, performance test results, and carry a limited warranty (typically 6–12 months). Always request proof of origin and refurbishment standards (e.g., ISO 13485 compliance) before procurement. |
Need a Quote for Dental Handpiece Manufacturers?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


