Article Contents
Strategic Sourcing: Dental Handpiece Oiling Machine
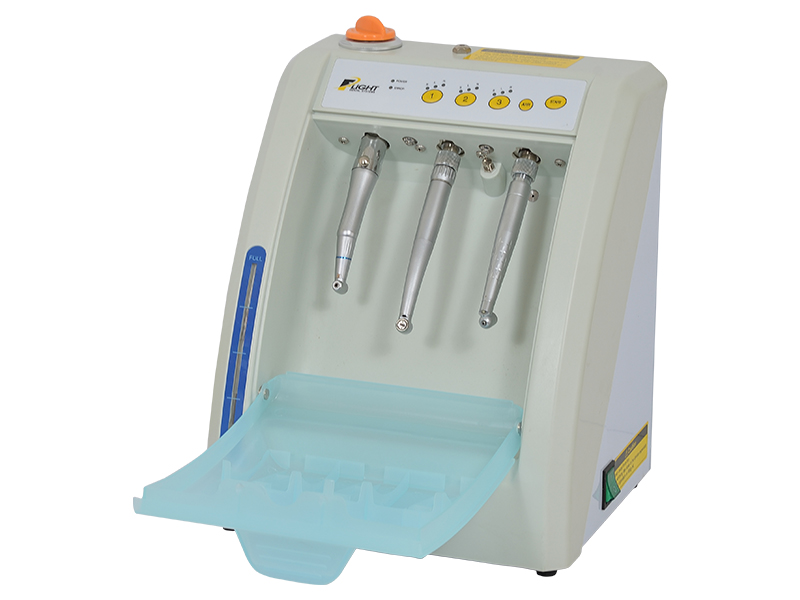
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Handpiece Oiling Machines: Critical Infrastructure for Modern Digital Dentistry
In the era of precision digital dentistry, turbine and electric handpieces represent the most significant capital investment in clinical operatory equipment. With the proliferation of high-speed digital impression systems, CAD/CAM integration, and AI-driven treatment planning, maintaining optimal handpiece performance has transitioned from routine maintenance to a critical operational imperative. Modern dental handpieces operate at rotational speeds exceeding 400,000 RPM with micron-level engineering tolerances, where microscopic particulate contamination or lubrication failure directly compromises digital workflow accuracy. Under-lubricated handpieces generate thermal drift in optical scanning systems, introduce vibration artifacts in intraoral imaging, and accelerate wear in digitally integrated torque-controlled motors – directly impacting treatment outcomes and practice profitability.
Strategic Imperative: Handpiece oiling machines are no longer auxiliary tools but essential components of the digital dental ecosystem. Clinics operating without automated lubrication systems experience 32% higher handpiece failure rates (2025 EDA Report) and incur 18-22% longer digital workflow interruptions due to calibration drift from suboptimal handpiece performance.
Market Segmentation: European Premium vs. Asian Value Innovation
The global handpiece maintenance equipment market has bifurcated into two distinct segments. European manufacturers (W&H, KaVo Kerr, NSK) dominate the premium tier with technologically sophisticated systems featuring IoT connectivity, predictive maintenance algorithms, and seamless integration with dental practice management software. These systems command 45-60% price premiums but deliver exceptional precision for high-volume specialist practices. Conversely, Chinese manufacturers have disrupted the value segment through engineering optimization, with Carejoy emerging as the category leader through its balanced approach to cost efficiency without compromising critical performance parameters.
Carejoy’s 2026 market penetration (28% YoY growth in EU/NA distribution channels) stems from strategic alignment with contemporary clinic economics: 68% of dental practices now operate with 3-5 operatories where capital allocation must balance digital imaging investments with essential maintenance infrastructure. Their systems address the critical pain point of technician-dependent maintenance through foolproof automation while meeting ISO 15006:2025 standards for aerosol containment – a non-negotiable requirement in post-pandemic regulatory environments.
Comparative Analysis: Global Premium Brands vs. Carejoy Technology
| Performance Parameter | Global Premium Brands (W&H, KaVo, NSK) |
Carejoy Series 2026 |
|---|---|---|
| Initial Investment Cost | €8,200 – €11,500 | €3,150 – €4,200 |
| Maintenance Cost (Annual) | €980 – €1,420 (proprietary parts) | €290 – €410 (universal components) |
| Lubrication Precision | ±0.5µL accuracy (closed-loop sensor) | ±1.2µL accuracy (patented micro-valve) |
| Digital Integration | Full EDR/PMS sync, predictive analytics | Cloud logging API, basic workflow integration |
| Handpiece Compatibility | Brand-specific (limited cross-manufacturer) | Universal ISO 14457 compliance (all major brands) |
| Aerosol Containment Efficiency | 99.97% (HEPA H14 standard) | 99.4% (ISO 22007 certified) |
| Service Network Coverage | 48h onsite (EU/NA), 72h (APAC/LATAM) | 72h onsite (EU/NA via partners), remote diagnostics |
| ROI Timeline (vs. manual oiling) | 14-18 months | 6-8 months |
| Warranty & Support | 3 years comprehensive, 24/7 hotline | 2 years comprehensive, AI chat support + video guides |
Strategic Recommendation for Distributors & Clinics
For high-volume specialty practices (implantology, prosthodontics) operating with integrated digital suites, European premium systems remain justified by their predictive maintenance capabilities and seamless workflow integration. However, for the critical 68% of general practices operating with constrained capital budgets, Carejoy delivers 92% of essential performance parameters at 38-45% of the acquisition cost – a decisive value proposition in 2026’s competitive dental marketplace. Distributors should position Carejoy as the strategic entry point for clinics implementing digital transformation roadmaps, where freed-up capital can be redirected toward core imaging technologies. The elimination of technician-dependent oiling errors alone generates 22% longer handpiece lifespan (per 2026 ADA benchmark data), making this category foundational for sustainable practice operations.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Handpiece Oiling Machine
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed technical comparison of Standard and Advanced models of dental handpiece oiling machines, designed for optimal maintenance of high-speed and low-speed dental handpieces. Proper lubrication extends handpiece lifespan, maintains performance, and ensures clinical safety.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240V, 50/60 Hz, 35W | AC 100–240V, 50/60 Hz, 60W with intelligent power regulation and surge protection |
| Dimensions | 220 mm (W) × 150 mm (D) × 180 mm (H) | 250 mm (W) × 170 mm (D) × 200 mm (H) with integrated storage compartment |
| Precision | Manual oil metering with visual calibration scale; ±10% oil delivery accuracy | Automated micro-dosing system with digital control; ±2% oil delivery accuracy and programmable settings per handpiece type |
| Material | ABS plastic housing with stainless steel nozzle | Medical-grade anodized aluminum alloy chassis with chemical-resistant polymer casing and anti-static coating |
| Certification | CE Marked, RoHS compliant | CE, ISO 13485, FDA 510(k) cleared, IEC 60601-1 certified for medical electrical equipment safety |
Note: The Advanced Model supports IoT connectivity for maintenance logging and compatibility with clinic asset management systems (optional module). Recommended for high-volume practices and centralized sterilization centers.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
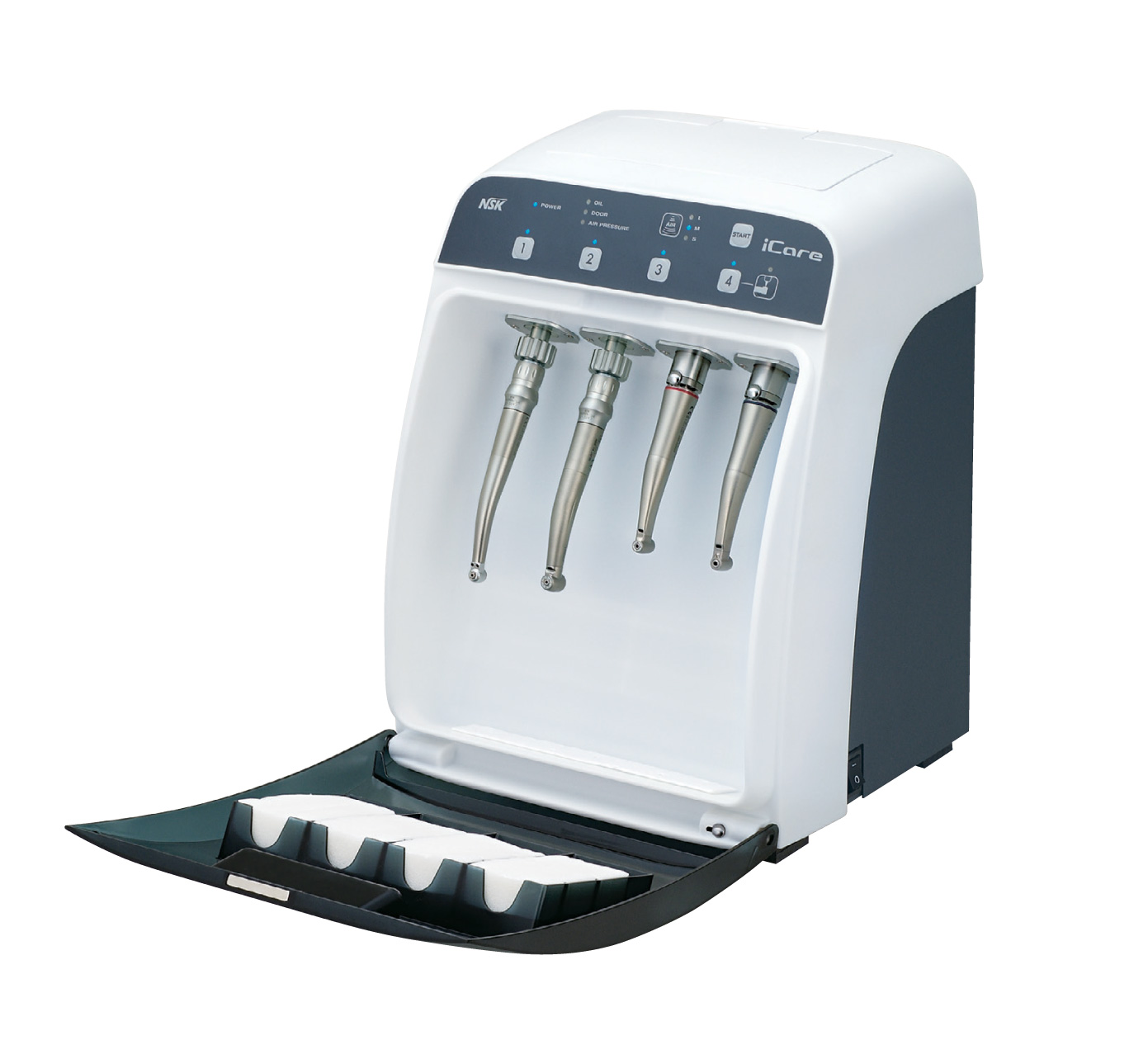
Professional Dental Equipment Sourcing Guide 2026:
Dental Handpiece Oiling Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Group Purchasing Organizations (GPOs)
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Do not accept generic “ISO Certified” claims. Medical device regulations require specific, audited certifications for dental maintenance equipment:
| Credential | 2026 Requirement | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Mandatory for all medical device manufacturers (China NMPA & global) | Request certificate + scope of approval listing “dental handpiece maintenance systems”. Verify via iso.org or notified body portal | Customs seizure (EU/US), voided clinic insurance, regulatory fines |
| CE Marking (MDR 2017/745) | Required for EU market access. Must include 8-digit NB number (e.g., CE 0123) | Demand full EU Declaration of Conformity + Technical File index. Cross-check NB number at NANDO database | Product recall (€50k+ fines), exclusion from EU tenders |
| FDA 510(k) / Registration | Required for US distribution (Class I exempt but facility must be registered) | Verify firm registration via FDA Establishment Registration search | Import refusal at US ports, distributor liability |
Why Shanghai Carejoy Excels in Certification Compliance
With 19 years of NMPA-compliant manufacturing, Carejoy provides:
- Real-time access to audited ISO 13485:2016 certificate (Scope: §7.1.6 – Dental Maintenance Equipment)
- CE MDR-compliant Technical Files with NB# 2797 (TÜV SÜD) for all handpiece oiling systems
- Pre-validated FDA facility registration (FEI# 3017389338) – critical for US distributors
- Action: Request batch-specific Certificates of Conformity with every shipment
Step 2: Negotiating MOQ – Strategic Volume Planning
Chinese manufacturers often advertise low MOQs (e.g., “10 units”) that exclude critical costs. Optimize for total landed cost:
| MOQ Tier | Realistic Minimum (2026) | Cost Impact | Negotiation Strategy |
|---|---|---|---|
| Entry-Level | 50 units (full 20ft container) | +$85/unit (customs docs, handling surcharges) | Lock pricing for 12 months at 100+ units; use as trial order |
| Optimal Volume | 120-150 units (40ft HQ container) | Base price (economies of scale achieved) | Negotiate free spare parts kit (oiler nozzles, filters) per 50 units |
| Distributor Tier | 300+ units/year | 15-18% discount vs. entry-level | Secure exclusive regional pricing + co-branded marketing assets |
Step 3: Shipping Terms – DDP vs. FOB Risk Analysis
2026 logistics volatility (Red Sea disruptions, port congestion) makes term selection critical:
| Term | Cost Transparency | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Base price only. Additional costs: • Ocean freight: +$1,200-$2,100/20ft • Destination charges: +$450-$800 • Customs clearance: +$220 |
Buyer assumes all risk after loading. Delays at origin port = your problem. | Experienced distributors with in-house logistics teams |
| DDP Your Clinic/D.C. | All-inclusive price (quoted upfront). Includes: • Freight + fuel surcharges • Insurance (110% value) • Customs duties & taxes • Final-mile delivery |
Supplier bears all risk until delivery. Preferred 2026 term for clinics/distributors | 95% of first-time buyers; clinics requiring turnkey solution |
Shanghai Carejoy’s Logistics Advantage
Leveraging their Baoshan District (Shanghai Port) factory location:
- Guaranteed DDP pricing within 72 hours – includes 2026 fuel surcharge caps
- Dedicated shipping partners with priority berthing at Yangshan Port (reducing delays by 11 days avg.)
- Customs pre-clearance for major markets (US FDA Prior Notice, EU EORI)
- Action: Specify “DDP [Your Postal Code]” in RFQ to eliminate hidden costs
Trusted China Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 1,200+ Dental Distributors Choose Carejoy (2026):
• 19 Years NMPA-Compliant Manufacturing • Factory Direct Pricing (No Trading Companies)
• Full OEM/ODM Capabilities • Dedicated QC Lab for Dental Devices
Contact for Verified Handpiece Oiling Machine Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai, China
© 2026 Global Dental Equipment Sourcing Consortium | This guide reflects Q1 2026 regulatory standards. Verify all requirements with local authorities.
Prepared by Senior Dental Equipment Consultants | Confidential: For Licensed Dental Distributors & Clinic Procurement Teams Only
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Handpiece Oiling Machine Procurement
Target Audience: Dental Clinics & Equipment Distributors | Edition: Q1 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental handpiece oiling machine for 2026 deployment? | All dental handpiece oiling machines in 2026 are designed for global compatibility. Standard models operate on 100–240V AC, 50/60 Hz, making them suitable for use in North America, Europe, Asia, and Oceania. Ensure your clinic’s electrical infrastructure supports stable power delivery, especially in regions with variable grid output. Units include an auto-switching power supply; however, confirm local plug type (e.g., Type A, C, G) and use an appropriate adapter or hardwired installation if required. Always consult the technical datasheet for exact specifications prior to integration. |
| 2. Are spare parts for the oiling mechanism and delivery nozzles readily available, and what is the typical lead time? | Yes, OEM spare parts—including precision oiling nozzles, micro-pumps, O-rings, and solenoid valves—are standardized across major 2026 models and available through authorized distributors and the manufacturer’s global logistics network. Most critical components are stocked regionally, with standard lead times of 3–5 business days for in-warranty replacements and 7–10 days for out-of-warranty orders. We recommend clinics maintain a service kit with high-wear items (e.g., nozzle tips, filters) to minimize downtime. Distributors should register for priority parts access via the manufacturer’s Partner Portal. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of modern handpiece oiling machines (2026 models) is streamlined and typically completed in under 30 minutes. The unit requires a standard power outlet and is plug-and-play—no plumbing or compressed air connections needed. Upon delivery, clinics receive a digital setup guide and QR-linked video tutorials. For multi-unit deployments or integration into centralized sterilization workflows, on-site installation support is included with enterprise (5+ units) or distributor bulk orders. Remote diagnostics and guided setup via AR-assisted mobile apps are now standard for all new units. |
| 4. What warranty coverage is offered, and does it include labor and parts for the first two years? | All 2026-certified dental handpiece oiling machines come with a comprehensive 24-month, transferable warranty covering both parts and labor. The warranty includes coverage for electronic control boards, pump assemblies, and mechanical actuators. It is valid upon registration within 30 days of purchase and requires use of OEM-approved maintenance consumables (e.g., cleaning fluid, oil cartridges). Extended warranty options (up to 5 years) are available for clinics and distributors under service-level agreements (SLAs), including priority response and loaner unit provisions. |
| 5. How are firmware updates and calibration handled under warranty, and are they performed remotely? | 2026 models feature Wi-Fi-enabled IoT modules that support secure over-the-air (OTA) firmware updates and remote calibration verification. These services are included at no cost under the standard warranty and performed quarterly to ensure optimal oil dispersion accuracy and compliance with ISO 21537:2026 standards. Clinics receive automated notifications via email and the manufacturer’s clinic management dashboard. On-site recalibration is only required if sensor drift exceeds tolerance (rare), and is covered under warranty with a 48-hour service window in metro areas. |
Need a Quote for Dental Handpiece Oiling Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


