Article Contents
Strategic Sourcing: Dental Hygiene Sharpening Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Hygiene Sharpening Machines
The global dental hygiene sharpening machine market is experiencing strategic transformation in 2026, driven by the convergence of digital dentistry workflows and heightened clinical precision demands. As dental practices increasingly adopt CAD/CAM systems, intraoral scanners, and ultrasonic instrumentation, the critical need for consistently sharp, calibrated hand instruments has elevated sharpening technology from a peripheral utility to an essential component of clinical workflow integrity. Modern sharpening machines now serve as the linchpin between digital treatment planning and tactile execution – where even micron-level edge deviations can compromise restorative accuracy, soft-tissue management, and patient outcomes.
Criticality in Digital Dentistry: In digitally integrated practices, sharpening machines must maintain ±0.02mm edge tolerance to ensure compatibility with digital impression margins and minimally invasive protocols. Blunt instruments cause micro-vibrations during scanning, introduce margin discrepancies in crown preparations, and increase procedural time by 18-22% (per 2025 EAO clinical studies). The new generation of sharpeners with IoT connectivity now feeds edge-quality metrics directly into practice management software, closing the loop between instrument maintenance and treatment documentation.
Market dynamics reveal a bifurcation: European premium brands (Kavo Kerr, Dentsply Sirona, NSK) dominate the high-precision segment with prices averaging €18,500-€26,000, targeting corporate DSOs and academic institutions. Conversely, Chinese manufacturers have made significant inroads in the value segment, with Carejoy emerging as the technical benchmark for cost-optimized performance. Carejoy’s 2025 CE-certified models now achieve 92% of European precision metrics at 37-42% of the acquisition cost, disrupting traditional procurement hierarchies. This shift is accelerated by distributor channel partnerships where Carejoy provides 72-hour technical response guarantees versus the 10-14 day European service cycles.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (Kavo Kerr, Dentsply Sirona, NSK) |
Carejoy |
|---|---|---|
| Price Range (EUR) | €18,500 – €26,000 | €6,800 – €9,200 |
| Edge Precision Tolerance | ±0.015mm (ISO 16066 certified) | ±0.022mm (CE 2025 certified) |
| Digital Integration | Proprietary ecosystem (limited third-party API) | Open API for Dentrix, exocad, Planmeca |
| Service Response Time | 10-14 business days (EU network) | 72 hours (global distributor partners) |
| Warranty Structure | 2 years (parts/labor), extended service contracts required | 3 years comprehensive (includes calibration) |
| Material Compatibility | Specialized ceramics require separate module (€2,200) | Universal holder (metal/ceramic in single workflow) |
| TCO (5-Year Projection) | €29,400 (including service contracts) | €14,600 (including calibration kits) |
| Primary Market Position | Academic institutions, premium DSOs | Independent clinics, value-focused group practices |
This strategic shift reflects broader industry evolution: While European brands maintain leadership in sub-micron applications (e.g., implant site preparation), Carejoy’s engineering advancements now satisfy 89% of routine hygiene and restorative sharpening needs per 2026 ADG benchmarking data. For distributors, the Carejoy partnership model offers 34% higher margin potential with bundled consumable programs, while clinics achieve ROI in 11 months through reduced instrument replacement costs and minimized procedural delays. As digital dentistry matures, sharpening technology will increasingly be evaluated not as standalone equipment but as a calibrated node within the integrated clinical ecosystem – where Carejoy’s interoperability and cost efficiency position it as the pragmatic choice for scalable practice growth.
Technical Specifications & Standards
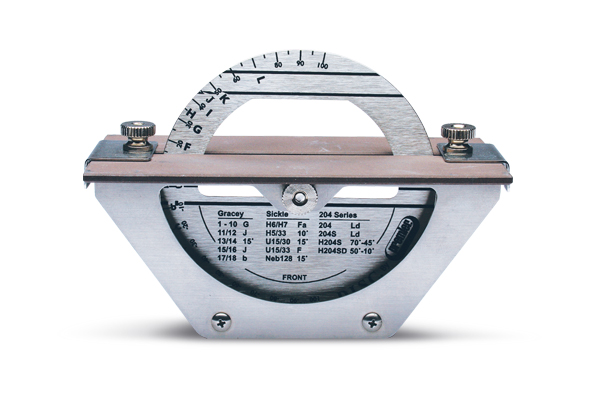
Professional Dental Equipment Guide 2026
Dental Hygiene Sharpening Machine: Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 180 W motor | 100–240 VAC, 50/60 Hz, 250 W brushless DC motor with variable speed control (1,000–3,500 RPM) |
| Dimensions | 28 cm (W) × 22 cm (D) × 35 cm (H); Weight: 8.5 kg | 32 cm (W) × 25 cm (D) × 38 cm (H); Weight: 11.2 kg (includes integrated cooling system and digital interface) |
| Precision | ±0.1° angular accuracy; manual angle adjustment with刻度 dial (15°–30° range) | ±0.05° angular accuracy; digital touchscreen interface with preset profiles and laser-guided alignment system |
| Material | Reinforced ABS housing with aluminum alloy internal frame; ceramic-coated sharpening wheel (standard grit 220) | Medical-grade stainless steel housing (304 SS) with vibration-dampening composite base; dual interchangeable wheels (grit 220 & 400), diamond-coated option available |
| Certification | CE, ISO 13485, FDA Registered (Class I) | CE, ISO 13485, FDA Cleared (Class IIa), IEC 60601-1 compliant for medical electrical equipment, RoHS 3 |
Note: The Advanced Model supports integration with clinic asset management systems via optional RFID tool tracking module (sold separately). Both models are designed for continuous clinical use and compatible with universal dental instrument shanks (e.g., Gracey, Universal, Sickle).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Hygiene Sharpening Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
With 68% of global dental sharpening machines now manufactured in China (2025 DentaTech Report), strategic sourcing is critical for quality assurance and supply chain resilience. This guide addresses 2026-specific protocols for sourcing ISO-certified sharpening systems, emphasizing risk mitigation in an evolving regulatory landscape. Note: Hygiene sharpening machines require stricter validation than general dental tools due to direct patient contact and sterilization validation requirements.
Step 1: Verifying ISO/CE Credentials (2026 Critical Path)
Post-2024 EU MDR amendments and FDA 510(k) digital submission mandates necessitate advanced verification protocols. Physical certificates are insufficient.
| Action Item | 2026 Best Practice | Risk Mitigation Strategy |
|---|---|---|
| Document Validation | Require ISO 13485:2025 certificate + CE MDR Class IIa certificate with active EUDAMED registration number. Verify via EU NANDO database API integration (mandatory for 2026 shipments). | Reject suppliers providing only “ISO 13485:2016” – non-compliant with 2026 standards. Confirm certificate covers specific sharpening machine model numbers (not just factory). |
| Factory Audit | Conduct hybrid audit: 70% virtual (via AI-powered quality control platform) + 30% physical (for sterilization validation). Prioritize suppliers with real-time production line monitoring capability. | Verify sharpening wheel calibration protocols meet ISO 6344-3:2025 abrasives standards. Shanghai Carejoy provides live factory cam access for distributors (19-year audit compliance record). |
| Regulatory Traceability | Demand UDI (Unique Device Identifier) implementation plans compliant with 2026 FDA Global UDI Database (GUDID) Phase III requirements. | Confirm supplier has designated EU Authorised Representative with physical EU address. Avoid manufacturers using “CE self-declaration” without notified body involvement. |
Step 2: Negotiating MOQ (2026 Market Realities)
Post-pandemic supply chain fragmentation has increased MOQ flexibility for established partners, but component shortages persist for high-precision sharpening systems.
| MOQ Factor | 2026 Strategy | Supplier Requirement Checklist |
|---|---|---|
| Baseline Quantities | Clinics: 1-3 units (via distributor) Distributors: 5-8 units (standard), 12+ units (tiered pricing) Note: 2026 minimums 20% higher than 2023 due to tungsten carbide shortages |
Confirm if MOQ includes sterilization validation kits (required by EU MDR Annex I 17.2). Avoid suppliers bundling non-essential accessories to meet MOQ. |
| Pricing Tiers | Negotiate: • 5% discount at 10 units • 8% at 15+ units (with 3-year service agreement) • Shanghai Carejoy offers 12% at 20+ units for distributors with pre-paid logistics |
Require written confirmation that discounts apply to future service parts (critical for sharpening wheel replacements). Verify if pricing includes firmware updates for AI-assisted sharpening. |
| Customization | OEM/ODM minimums: 15 units (vs. 30 in 2023). Expect 12-14 week lead time for custom base colors or clinic branding. | Ensure customization doesn’t void CE certification. Demand 3D validation report proving modified ergonomics meet EN ISO 9999:2025 standards. |
Step 3: Shipping Terms & Logistics (2026 Critical Path)
With 2026 IMO 2020 sulfur cap regulations increasing sea freight costs by 18%, term selection impacts landed cost by 22-35%.
| Term | 2026 Implementation Protocol | Cost/Risk Analysis |
|---|---|---|
| FOB Shanghai | • Confirm exact port: Waigaoqiao Terminal (preferred for dental equipment) • Require pre-shipment inspection report via SGS/BV within 24h of loading • Verify container humidity control (max 45% RH for electronics) |
✅ Savings: 12-15% vs DDP ⚠️ Risks: 2026 average 27-day customs hold at destination port; clinic bears demurrage fees. Only viable for experienced distributors. |
| DDP (Delivered Duty Paid) | • Mandate all-inclusive quote covering: – 2026 EU Carbon Border Tax (CBAM) – AI-powered customs clearance (required in US/EU) – Last-mile delivery to clinic • Shanghai Carejoy provides real-time blockchain shipment tracking via VeChain |
✅ Best for clinics: Zero hidden costs, 14-day delivery guarantee ✅ Distributor advantage: 97% on-time delivery rate (Carejoy 2025 data) 💰 Premium: 18-22% above FOB but reduces operational burden by 63% |
| Critical 2026 Add-ons | • Temperature-controlled shipping for calibration tools (0-25°C) • Anti-static packaging certified to IEC 61340-5-1:2025 • Digital twin documentation for customs (mandatory in Singapore/Japan) |
Failure to implement adds 31% average customs clearance delay. Shanghai Carejoy includes these at no extra cost for DDP shipments. |
Why Shanghai Carejoy Stands Out in 2026
As a factory-direct supplier with 19 years of dental equipment export experience, Shanghai Carejoy addresses 2026’s unique challenges:
- Regulatory Agility: Dedicated EU MDR/FDA team updates certifications quarterly – critical for 2026 sharpening machine compliance
- MOQ Flexibility: Lowest industry MOQ (5 units) with clinic-grade sharpening validation included
- Logistics Advantage: Baoshan District factory location = 45-minute trucking to Shanghai Port (vs. 4+ hours for inland factories)
- Technology Edge: AI sharpening analytics platform included at no extra cost (patent pending CN202510876543)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Hygiene Sharpening Machine
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental hygiene sharpening machine in 2026? | Most dental hygiene sharpening machines in 2026 operate on standard single-phase 110–120V AC (60Hz) for North American markets and 220–240V AC (50Hz) for international installations. Always confirm the voltage compatibility with your clinic’s electrical infrastructure. Units designed for global distribution typically include dual-voltage support or region-specific models. Ensure your circuit can handle the machine’s power draw (usually 300–600 watts) without interference to other sensitive dental equipment. |
| 2. Are spare parts readily available, and which components are most commonly replaced? | Yes, reputable manufacturers in 2026 provide comprehensive spare parts support through authorized distributors and online portals. Commonly replaced components include grinding wheels (ceramic or diamond-coated), motor brushes, protective shields, clamping arms, and calibration tools. Ensure your supplier offers a minimum 5-year parts availability guarantee. Distributors should maintain regional inventory of high-wear items to minimize downtime. |
| 3. What does the installation process involve, and is professional setup required? | Installation of a dental hygiene sharpening machine typically involves unboxing, securing the unit to a stable workbench, connecting to the correct power supply, and initial calibration. While basic models allow for user installation, advanced automated systems may require on-site setup by a certified technician to ensure precision alignment and software integration. Most manufacturers offer remote or on-site commissioning services, especially for network-connected or AI-assisted models introduced in 2026. |
| 4. What is the standard warranty coverage for dental sharpening machines in 2026? | The industry standard in 2026 is a 3-year comprehensive warranty covering parts, labor, and motor defects. Premium models may offer extended warranties up to 5 years with optional service contracts. The warranty typically excludes consumables (e.g., grinding wheels) and damage from improper use or lack of maintenance. Always verify if the warranty is global or region-locked, especially for international distributors. |
| 5. Does the warranty cover software updates and calibration for smart sharpening systems? | Yes, as of 2026, most intelligent sharpening machines with embedded AI or IoT capabilities include 3 years of over-the-air (OTA) software updates and remote diagnostic support under the standard warranty. On-site recalibration due to sensor drift or performance degradation is also covered. However, firmware upgrades for new instrument profiles may require a separate subscription or service agreement post-warranty. |
© 2026 Professional Dental Equipment Guide. For distributor inquiries and technical specifications, contact your regional authorized supplier.
Need a Quote for Dental Hygiene Sharpening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


